Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all 5 I really need the answers :) 16. If the pressure exerted on a confined gas is halved, then the volume of
please answer all 5 I really need the answers :) 
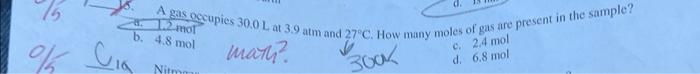
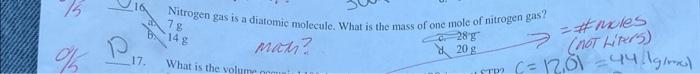
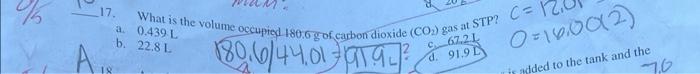

16. If the pressure exerted on a confined gas is halved, then the volume of the gas Increases four times Decreases by one-fourth d. Is halved 6. A gas oecupies 30.0L at 3.9atm and 27.C. How many moles of gas are present in the sample? b. 4.8mol marn? c. 2.4mol d. 6.8mol Nitrogen gas is a diatomic molecule. What is the mass of one mole of nitrogen gas? (4) 7g 14g man? ch 28g = ANxles 17 What is the volum (not hiper's) 17. a. 0.439L b. 22.8L. 20. A balloon is filled with 3.8L of helium gas at STP. Approximately how many moles of helium are contained in the balloon? b.0.17mol0.26mol 
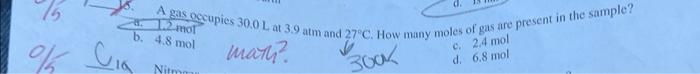
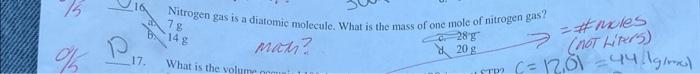
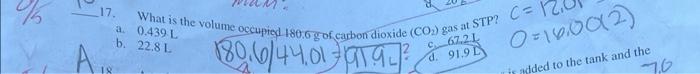

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


