Question
PLEASE ANSWER ALL! Choose the right one. Explain in one sentence. 1.Diffusion through the narrow pores of a catalyst pellet would enhance reaction. A: True
PLEASE ANSWER ALL! Choose the right one. Explain in one sentence.
1.Diffusion through the narrow pores of a catalyst pellet would enhance reaction. A: True B: False
2. Knudsen diffusion occurs when the pellet pore diameter is smaller than molecule mean free path. A: True B: False
3. Activated, configurational diffusion occurs usually when pellet pore diameter is A: > 100 nm B: 4-100 nm C:
4. In the pellet, the actual path that molecule travels from A to B is shown in following figure. How much is the tortuosity? 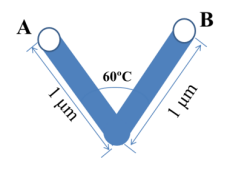
A: 0.5 B: 1 C: 2 D: 3
5. What is the ratio of effective diffusivity (in the pellet) to bulk diffusivity, if the tortuosity is 4 and the pellet porosity is 0.5? A: 1/4 B: 1/8 C: 4 D: 8
6. When the Thiele Modulus is very large, which from the cartoons below represents better reactant the concentration of A (blue) in the catalyst pellet?

7. When the Thiele Modulus is very small, which from the cartoons below represents better reactant the concentration of A (blue) in the catalyst pellet?

8. For a first-order reaction, with internal mass transfer resistances, how would the observed reaction rate change if the pellet diameter was increased? A: Increase B: No change C: Decrease
9. For a first-order reaction, how would the observed reaction rate change if the reaction temperature was increased? A: Increase B: No change C: Decrease
10. For a second-order reaction, with internal mass transfer resistances, how would the observed reaction rate change if the reactant concentration at the external pellet surface decreased? A: Increase B: No change C: Decrease
11. For a reaction limited by internal diffusion, how would the reaction rate change if the fluid velocity was increased? A: Increase B: No change C: Decrease
12. For a reaction limited by internal diffusion, how would the observed reaction rate change if the pellet diameter was decreased? A: Increase B: No change C: Decrease
13. For a reaction limited by surface reaction, how would the observed reaction rate change if the fluid velocity was increased? A: Increase B: No change C: Decrease
14. For a reaction limited by surface reaction, how would the observed reaction rate change if the pellet diameter was increased? A: Increase B: No change C: Decrease
15. If internal effectiveness factor () is close to 1, the reaction is controlled by: A: Surface reaction B: internal diffusion C: Surface reaction and internal diffusion
16. If internal effectiveness factor () is 0.1, the reaction is controlled by: A: Surface reaction B: Internal diffusion C: Surface reaction and internal diffusion
17. If the Weisz-Prater parameter CWP >> 1, reactant concentration gradient within the pellet is A: Large B: Small C: Goes through a maximum
18. If the Weisz-Prater parameter CWP
19. For a first-order reaction, if Thiele modulus is 2 and internal effectiveness factor () is 0.8, whats the value of Weisz-Prater parameter? A: 0.4 B: 0.8 C: 1.6 D: 3.2
A: B: C: A: B: CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


