Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all part of question 5 and the gollow up questions thank you 5) Using Chapter C in your textbook to help you: a.
please answer all part of question 5 and the gollow up questions 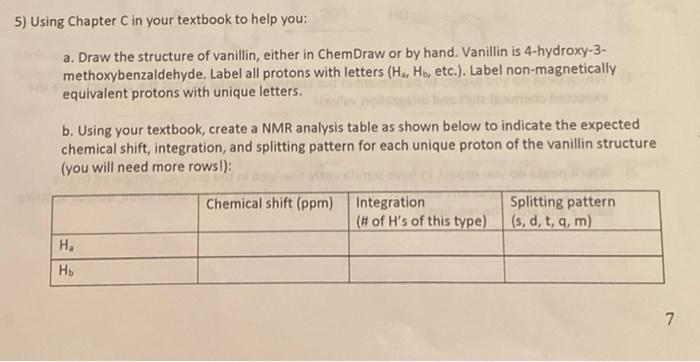
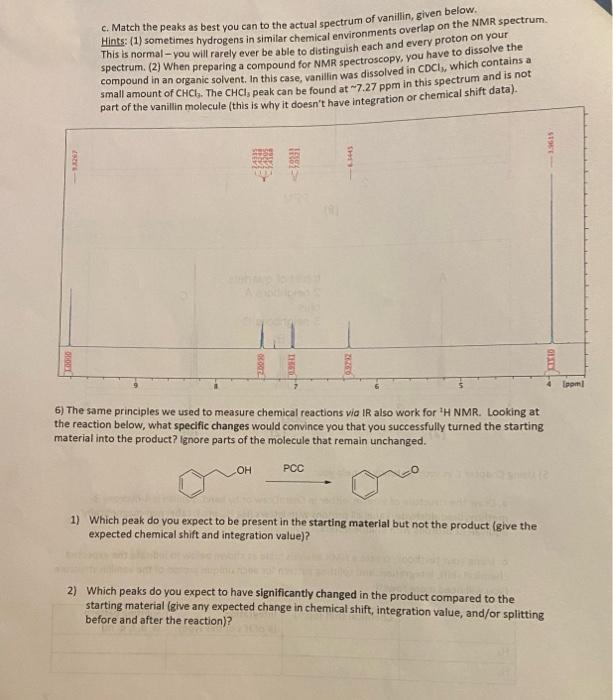
5) Using Chapter C in your textbook to help you: a. Draw the structure of vanillin, either in ChemDraw or by hand. Vanillin is 4-hydroxy-3- methoxybenzaldehyde. Label all protons with letters (H., H., etc.). Label non-magnetically equivalent protons with unique letters. b. Using your textbook, create a NMR analysis table as shown below to indicate the expected chemical shift, integration, and splitting pattern for each unique proton of the vanillin structure (you will need more rows!): Chemical shift (ppm) Integration (# of H's of this type) Splitting pattern (s, d, t, q, m) H. 7 C. Match the peaks as best you can to the actual spectrum of vanillin, given below. Hints: (1) sometimes hydrogens in similar chemical environments overlap on the NMR spectrum spectrum. (2) When preparing a compound for NMR spectroscopy, you have to dissolve the This is normal - you will rarely ever be able to distinguish each and every proton on your compound in an organic solvent. In this case, vanillin was dissolved in CDC, which contains a small amount of CHCI.. The CHCI, peak can be found at 7.27 ppm in this spectrum and is not part of the vanillin molecule (this is why it doesn't have integration or chemical shift data). SE 5831 OLTO Laam! 6) The same principles we used to measure chemical reactions via IR also work for 'H NMR. Looking at the reaction below, what specific changes would convince you that you successfully turned the starting material into the product? Ignore parts of the molecule that remain unchanged. OH PCC 1) Which peak do you expect to be present in the starting material but not the product (give the expected chemical shift and integration value)? 2) Which peaks do you expect to have significantly changed in the product compared to the starting material (give any expected change in chemical shift, integration value, and/or splitting before and after the reaction) thank you


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


