Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all parts Acetylene (C2H2) gas and oxygen (O2) gas react to form carbon dioxide (CO2) gas and water (H2O) vapor. Suppose you have
please answer all parts 
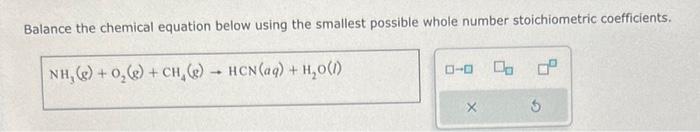
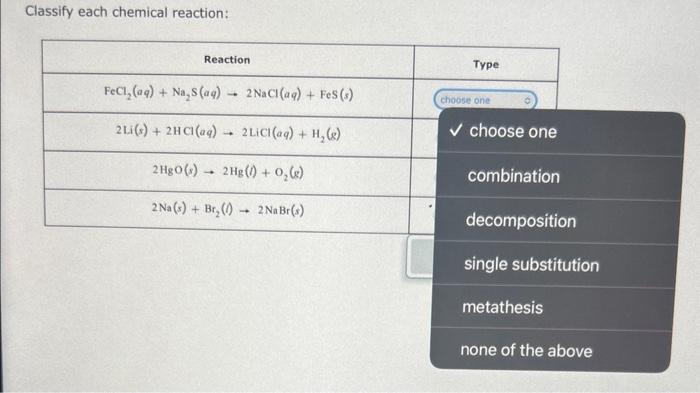
Acetylene (C2H2) gas and oxygen (O2) gas react to form carbon dioxide (CO2) gas and water (H2O) vapor. Suppose you have 13.0mol of C2H2 and 3.0mol of O2 in a reactor: Calculate the largest amount of CO2 that could be produced. Round your answer to the nearest 0.1 mol. Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. NH3(g)+O2(g)+CH4(g)HCN(aq)+H2O(l) Classify each chemical reaction 
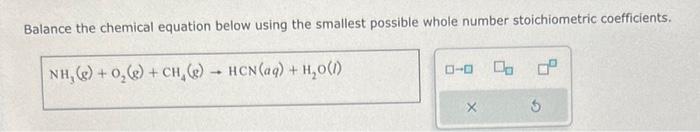

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


