Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all parts fully and show all work, thank you so much! 2. Given the following aqueous acid solution. a. Place the acids in
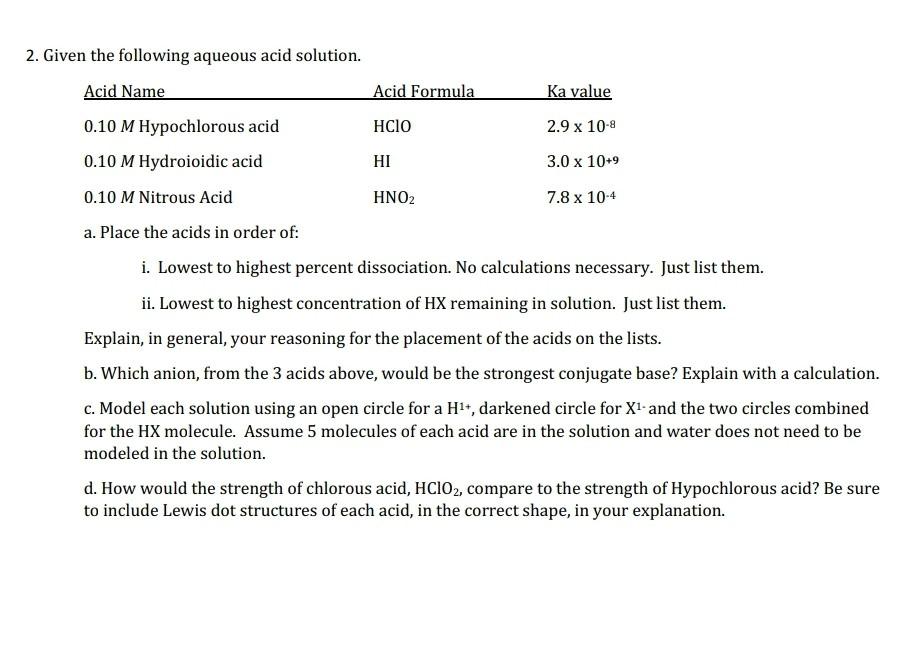
please answer all parts fully and show all work, thank you so much!
2. Given the following aqueous acid solution. a. Place the acids in order of: i. Lowest to highest percent dissociation. No calculations necessary. Just list them. ii. Lowest to highest concentration of HX remaining in solution. Just list them. Explain, in general, your reasoning for the placement of the acids on the lists. b. Which anion, from the 3 acids above, would be the strongest conjugate base? Explain with a calculation. c. Model each solution using an open circle for a H1+, darkened circle for X1 and the two circles combined for the HX molecule. Assume 5 molecules of each acid are in the solution and water does not need to be modeled in the solution. d. How would the strength of chlorous acid, HClO2, compare to the strength of Hypochlorous acid? Be sure to include Lewis dot structures of each acid, in the correct shape, in your explanationStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


