please answer all parts in a clear handwriting
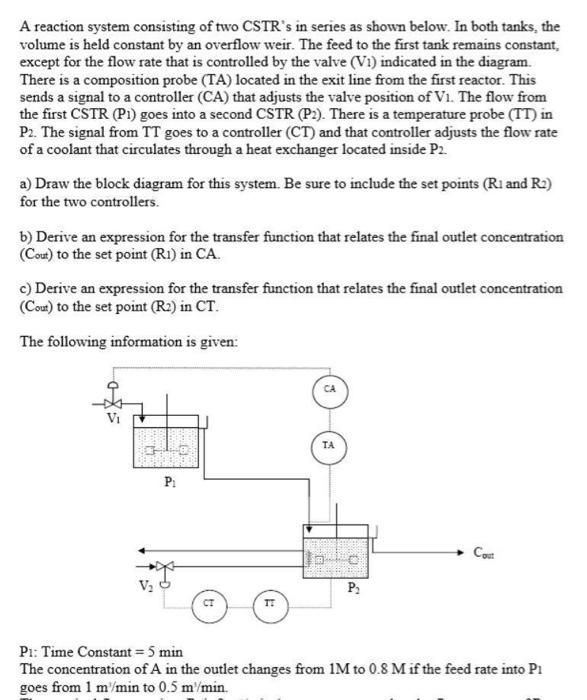
A reaction system consisting of two CSTR's in series as shown below. In both tanks, the volume is held constant by an overflow weir. The feed to the first tank remains constant, except for the flow rate that is controlled by the valve (V1) indicated in the diagram. There is a composition probe (TA) located in the exit line from the first reactor. This sends a signal to a controller (CA) that adjusts the valve position of V1. The flow from the first CSTR (P1) goes into a second CSTR (P2). There is a temperature probe (TT) in P2. The signal from TT goes to a controller (CT) and that controller adjusts the flow rate of a coolant that circulates through a heat exchanger located inside P2 a) Draw the block diagram for this system. Be sure to include the set points (R1 and R2) for the two controllers. b) Derive an expression for the transfer function that relates the final outlet concentration (Cout) to the set point (R1) in CA. c) Derive an expression for the transfer function that relates the final outlet concentration (Cout) to the set point (R2) in CT. The following information is given: P1: Time Constant =5min The concentration of A in the outlet changes from 1M to 0.8M if the feed rate into P1 goes from 1m1/min to 0.5m1/min. P1: Time Constant =5min The concentration of A in the outlet changes from 1M to 0.8M if the feed rate into P1 The nominal flow rate into P1 is 2m1/min (you may assume that the flow rate out of P1 and into P2 is the same as this value). The volume of the transfer line between the exit from P1 to the probe (TA) is 0.5m.. P2: Time Constant =10min The concentration in the outlet from P2 changes from 1M to 0.6M if the temperature in P2 changes from 100C to 140C. V1: It takes 0.8 minutes for valve 1 to essentially reach its new position after a change in input signal. controller CA changes from 75% to 25%. V2: It takes 0.5 minutes for valve 2 to essentially reach its new position after a change in input signal. The flow rate through valve 2 changes from 10m/min to 8m1/min when the signal from controller CT changes from 40% to 60%. TA: This probe reacts instantaneously to any change in composition. For a change in composition from 1M to 0.8M the probe output changes from 16mA to 12mA. TT: This probe also reacts instantaneously to any change in temperature. For a temperature change from 100C to 140C the output from the probe changes from 10mA to 12mA. CA: This is a PI controller with an integral time of 3 minutes and a gain of 10%/mA. CT: This is a PI controller with an integral time of 5 mintues and a gain of 5%/mA. d) Is this system stable for changes in R1 and R2 ? e) If this reaction is exothermic would you use a fail-open (air-to-close) or fail-close (airto-open) valve for V1 ? Why? f) Would you use a fail-open or fail-close valve for V V2? Why