Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all parts of the question correctly for a thumbs up The amino acid glycine is often used as the main ingredient of a
please answer all parts of the question correctly for a thumbs up 
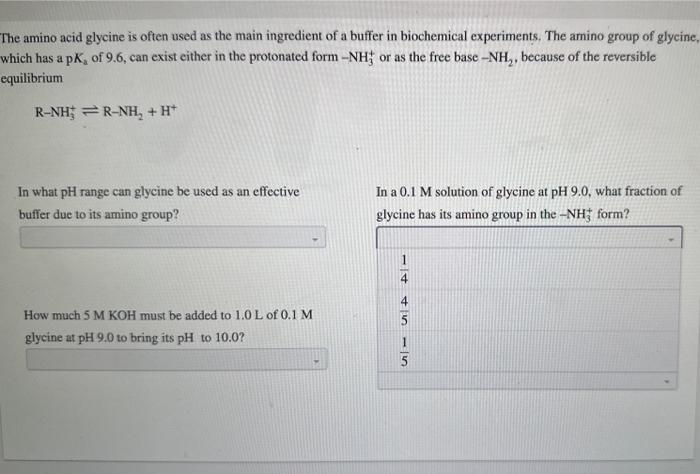
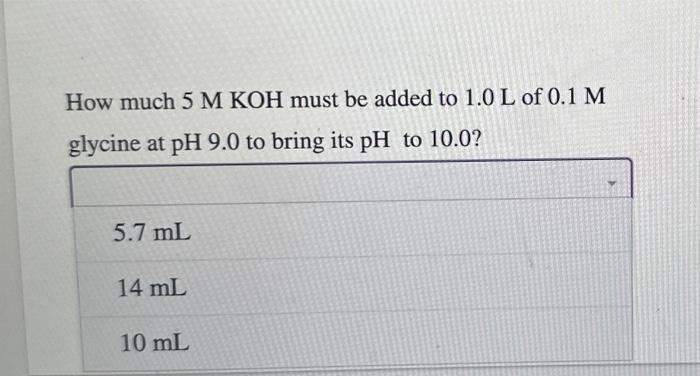
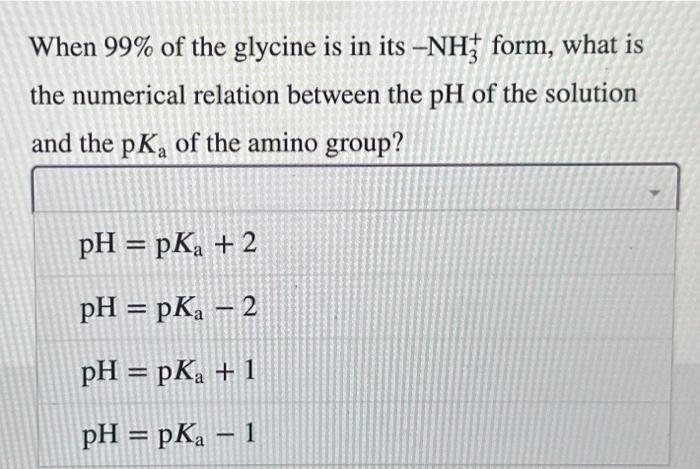
The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a p Ka of 9.6, can exist either in the protonated form NH3+or as the free base NH2, because of the reversible equilibrium RNH3+RNH2+H+ In what pH range can glycine be used as an effective In a 0.1M solution of glycine at pH9.0, what fraction of buffer due to its amino group? glycine has its amino group in the NH3+form? When 99% of the glycine is in its NH3+form, what is the numerical relation between the pH of the solution and the pKa of the amino group? The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a pKa of 9.6, can exist either in the protonated form NH3+or as the free base NH2, because of the reversible equilibrium RNH3+RNH2+H+ In what pH range can glycine be used as an effective In a 0.1 M solution of glycine at pH9.0, what fraction of buffer due to its amino group? glycine has its amino group in the NH3+form? How much 5MKOH must be added to 1.0L of 0.1M glycine at pH9.0 to bring its pH to 10.0 ? How much 5MKOH must be added to 1.0L of 0.1M glycine at pH9.0 to bring its pH to 10.0 ? When 99% of the glycine is in its NH3+form, what is the numerical relation between the pH of the solution and the pKa of the amino group? pH=pKa+2pH=pKa2pH=pKa+1pH=pKa1 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


