Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer all questions and ASAP. They have been asked already on Chegg and I've received the incorrect answers. I've included the hints given after
Please answer all questions and ASAP. They have been asked already on Chegg and I've received the incorrect answers. I've included the hints given after I had gotten them incorrect. I am still stuck on these ones. I only have one more chance to answer these questions so I would greatly appreciate your best efforts on solving these.
Just please answer as many as possible. I will give upvote. Thanks!
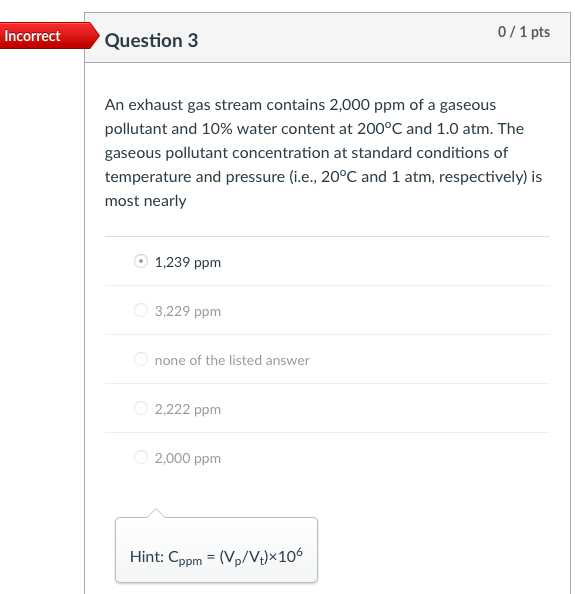
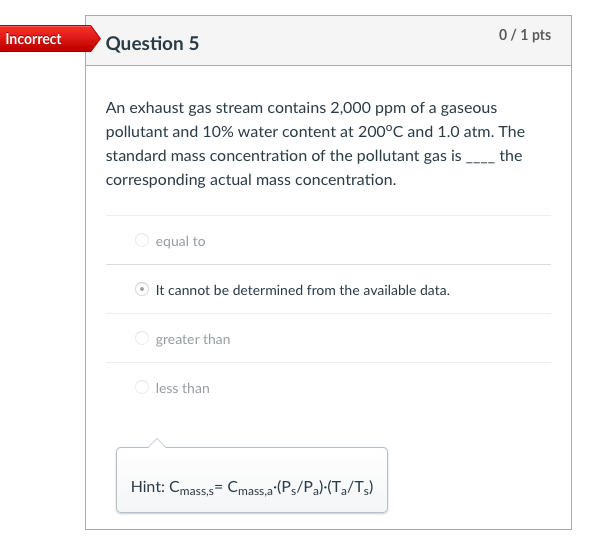
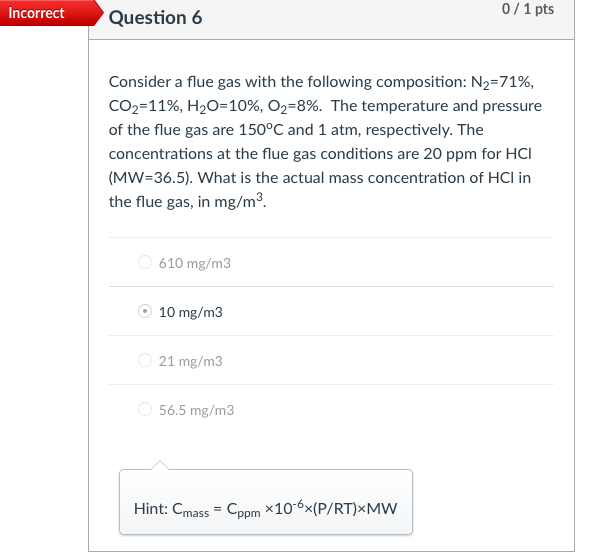
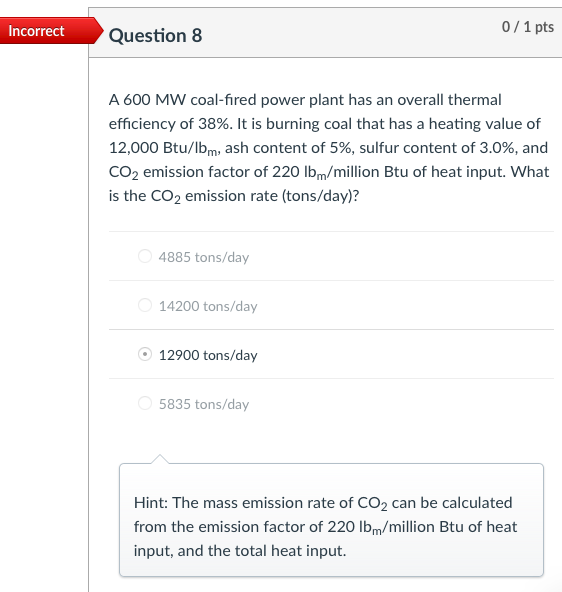
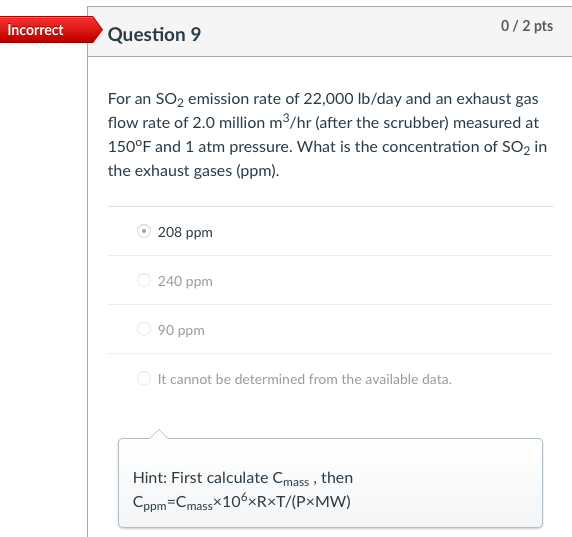
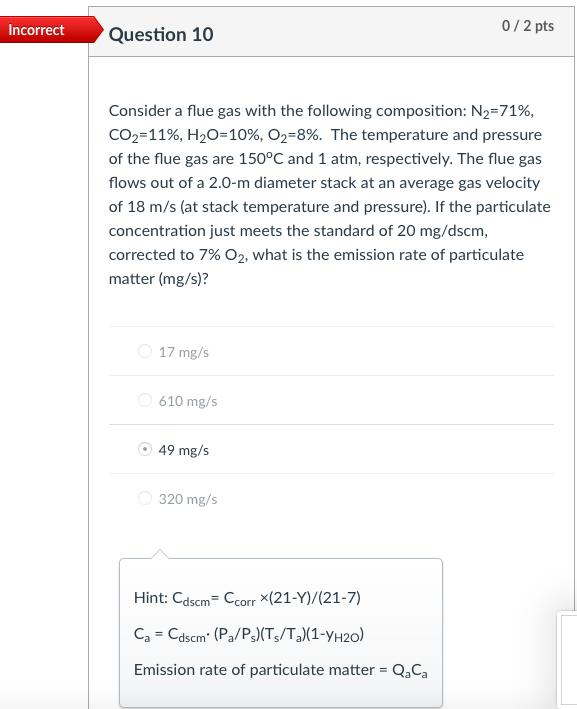
Incorrect Question 3 0/1 pts An exhaust gas stream contains 2,000 ppm of a gaseous pollutant and 10% water content at 200C and 1.0 atm. The gaseous pollutant concentration at standard conditions of temperature and pressure (i.e., 20C and 1 atm, respectively) is most nearly 1,239 ppm 3,229 ppm none of the listed answer 2,222 ppm 2,000 ppm Hint: Cppm = (v2/V+)x106 Incorrect Question 5 0/1 pts An exhaust gas stream contains 2,000 ppm of a gaseous pollutant and 10% water content at 200C and 1.0 atm. The standard mass concentration of the pollutant gas is ____ the corresponding actual mass concentration. equal to It cannot be determined from the available data. greater than less than Hint: Cmass,s= Cmass,a:(P3/P2)-(T2/T3) Incorrect 0/1 pts Question 6 Consider a flue gas with the following composition: N2=71%, CO2=11%, H20=10%, O2=8%. The temperature and pressure of the flue gas are 150C and 1 atm, respectively. The concentrations at the flue gas conditions are 20 ppm for HCI (MW=36.5). What is the actual mass concentration of HCl in the flue gas, in mg/m? 610 mg/m3 10 mg/m3 21 mg/m3 56.5 mg/m3 Hint: Cmass = Cppm *10-6x(P/RT)xMW Incorrect Question 8 0/1 pts A 600 MW coal-fired power plant has an overall thermal efficiency of 38%. It is burning coal that has a heating value of 12,000 Btu/lbm, ash content of 5%, sulfur content of 3.0%, and CO2 emission factor of 220 lbm/million Btu of heat input. What is the CO2 emission rate (tons/day)? 4885 tons/day 14200 tons/day 12900 tons/day 5835 tons/day Hint: The mass emission rate of CO2 can be calculated from the emission factor of 220 lbm/million Btu of heat input, and the total heat input. Incorrect 0/2 pts Question 9 For an SO2 emission rate of 22,000 lb/day and an exhaust gas flow rate of 2.0 million m3/hr (after the scrubber) measured at 150F and 1 atm pressure. What is the concentration of SO2 in the exhaust gases (ppm). 208 ppm 240 ppm 90 ppm It cannot be determined from the available data. Hint: First calculate Cmass, then Cppm=Cmass*106xRxT/(PxMW) Incorrect 0/2 pts Question 10 Consider a flue gas with the following composition: N2=71%, CO2=11%, H20=10%, 02=8%. The temperature and pressure of the flue gas are 150C and 1 atm, respectively. The flue gas flows out of a 2.0-m diameter stack at an average gas velocity of 18 m/s (at stack temperature and pressure). If the particulate concentration just meets the standard of 20 mg/dscm, corrected to 7% O2, what is the emission rate of particulate matter (mg/s)? 17 mg/s 610 mg/s 49 mg/s 320 mg/s Hint: Cdscm= Ccorr (21-Y)/(21-7) Ca = Cdscm: (P/PT3/T2/(1-YH20) Emission rate of particulate matter = Q. Ca
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


