Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer all questions thank you! View Policies Current Attempt in Progress Write an equation for the proton transfer reaction that occurs when the following
Please answer all questions thank you! 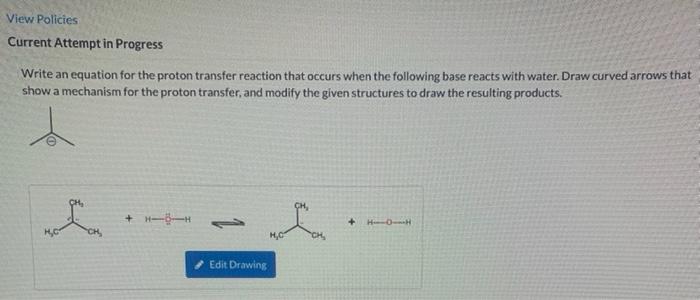
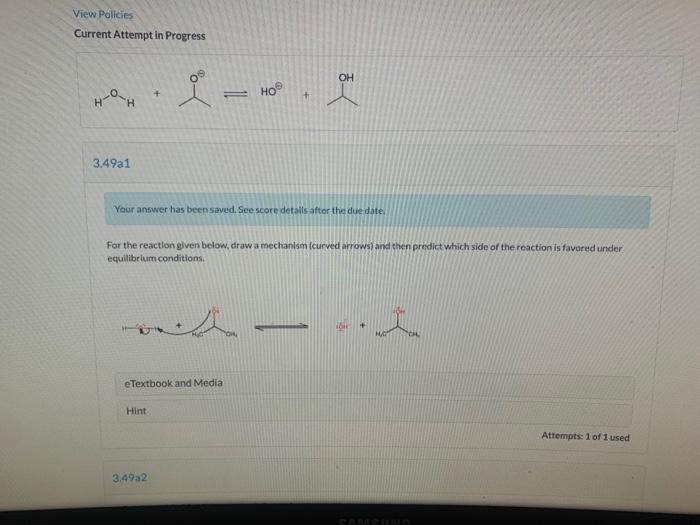
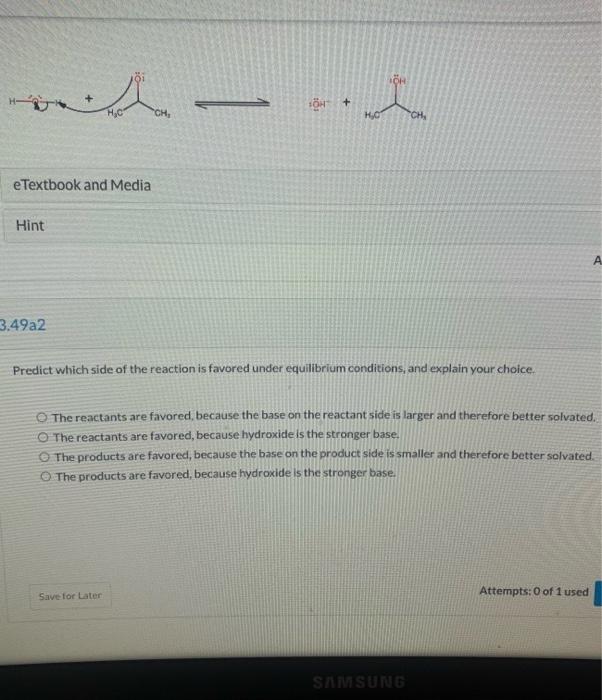
View Policies Current Attempt in Progress Write an equation for the proton transfer reaction that occurs when the following base reacts with water. Draw curved arrows that show a mechanism for the proton transfer, and modify the given structures to draw the resulting products. CH + -- + HO CH, HC Edit Drawing View Policies Current Attempt in Progress OH - + 3.49a1 Your answer has been saved. See score details after the due date, For the reaction given below, draw a mechanism (curved arrows) and then predict which side of the reaction is favored under equilibrium conditions. e Textbook and Media Hint Attempts: 1 of 1 used 3,49a2 OH H + CH HO CH, e Textbook and Media Hint 3.49a2 Predict which side of the reaction is favored under equilibrium conditions, and explain your choice. The reactants are favored, because the base on the reactant side is larger and therefore better solvated. O The reactants are favored, because hydroxide is the stronger base. The products are favored, because the base on the product side is smaller and therefore better solvated. The products are favored, because hydroxide is the stronger base. Save for Later Attempts: 0 of 1 used SAMSUNG 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


