Answered step by step
Verified Expert Solution
Question
1 Approved Answer
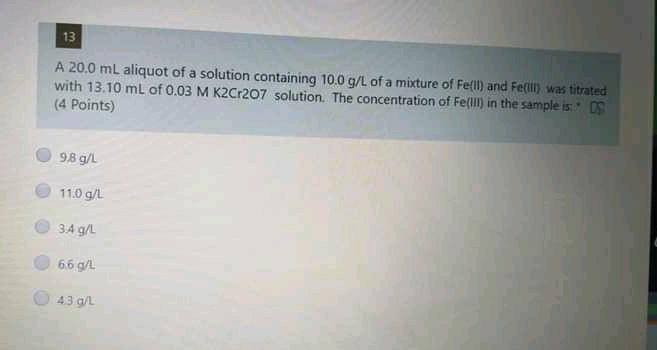
Please answer all the 4 sub question 13 A 20.0 mL aliquot of a solution containing 10.0 g/L of a mixture of Fe{ll) and Fe(ill)
Please answer all the 4 sub question
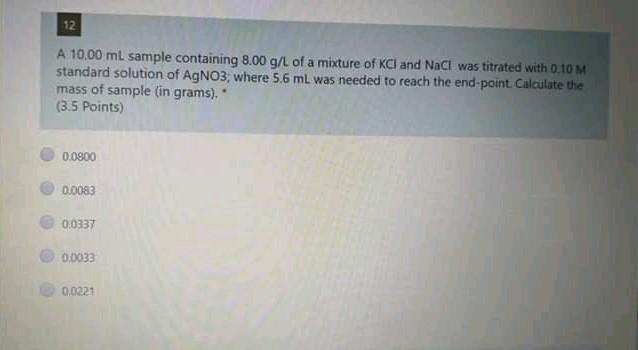
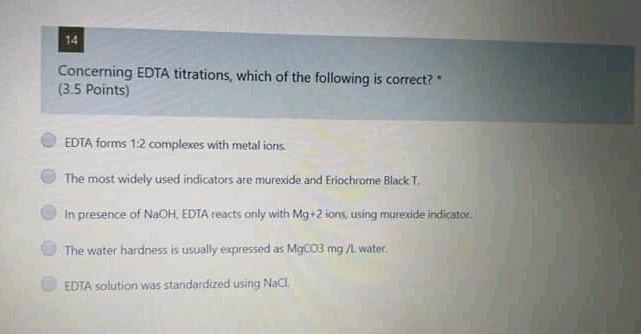
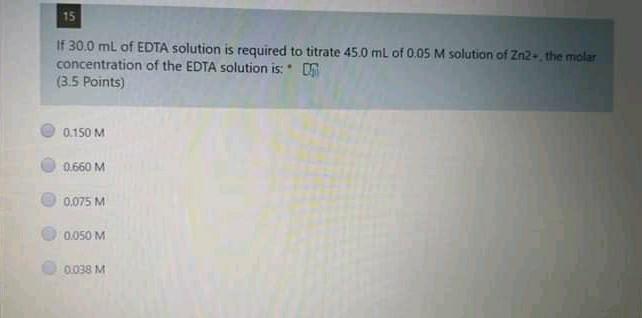
13 A 20.0 mL aliquot of a solution containing 10.0 g/L of a mixture of Fe{ll) and Fe(ill) was titrated with 13.10 mL of 0.03 M K2Cr207 solution. The concentration of Fell in the sample is OS (4 Points) 98 g/L 11.0 g/L. 3.4 g/L 66 g/L. 43 g/L 12 A 10.00 ml sample containing 8.00 g/L of a mixture of KCl and NaCl was titrated with 0.10 M standard solution of AgNO3, where 5.6 mL was needed to reach the end-point. Calculate the mass of sample (in grams). (3.5 points) 0.0800 010083 0:0337 0.0033 0.0221 14 Concerning EDTA titrations, which of the following is correct? (3.5 Points) EDTA forms 12 complexes with metal ions. The most widely used indicators are murexide and Eriochrome Black T. In presence of NaOH, EDTA reacts only with Mg+2 tons, using murexide indicator The water hardness is usually expressed as MgCO3 mg/L water. EDTA solution was standardized using Nag 15 If 30.0 mL of EDTA solution is required to titrate 45.0 mL of 0.05 M solution of Zn2. the molar concentration of the EDTA solution is: (3.5 Points) 0.150 M 0.660 M 0,075 M 0.050 M 1.038 MStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


