Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all the question clearly. thank you very much The following reaction occurs in a isothermal, ideal reaction, plug flow reactor: C4 H2O3 +
please answer all the question clearly. thank you very much 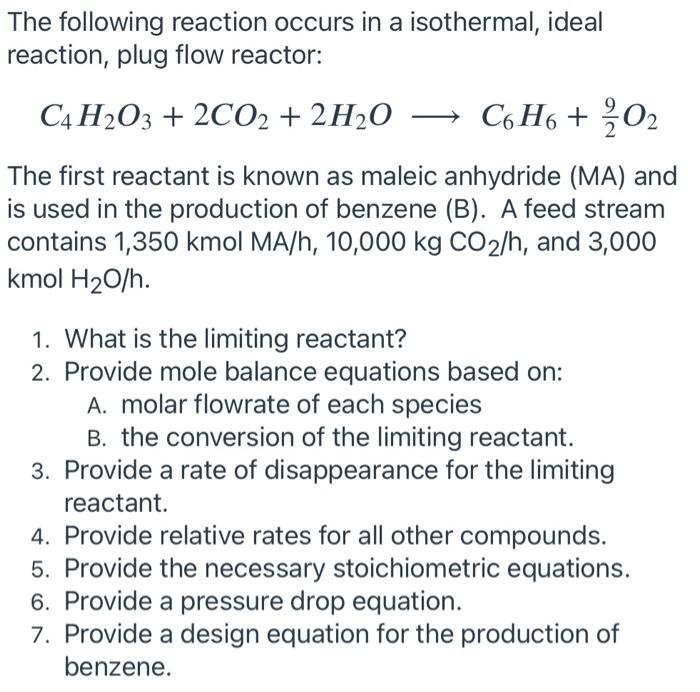
The following reaction occurs in a isothermal, ideal reaction, plug flow reactor: C4 H2O3 + 2CO2 + 2H20 C6H6 + ?O2 The first reactant is known as maleic anhydride (MA) and is used in the production of benzene (B). A feed stream contains 1,350 kmol MA/h, 10,000 kg CO2/h, and 3,000 kmol H20/h. 1. What is the limiting reactant? 2. Provide mole balance equations based on: A. molar flowrate of each species B. the conversion of the limiting reactant. 3. Provide a rate of disappearance for the limiting reactant. . 4. Provide relative rates for all other compounds. 5. Provide the necessary stoichiometric equations. 6. Provide a pressure drop equation. 7. Provide a design equation for the production of benzene 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


