Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all the questions Which of the following gases would have the greatest density at STP? Question 3 Calculate the density of methane gas
please answer all the questions 

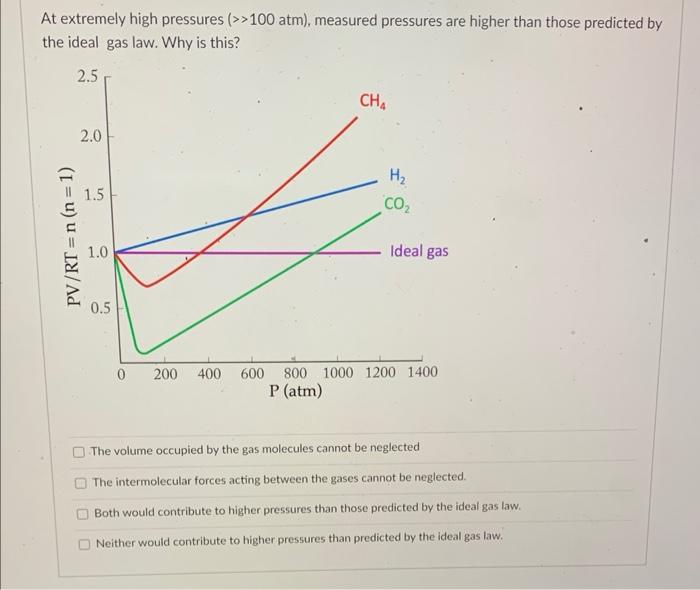
Which of the following gases would have the greatest density at STP? Question 3 Calculate the density of methane gas (CH4, units of g/L) at 290K and 120kPa. Note: Canvas will not allow you to enter units, please enter only your numerical value. Water can be broken down into hydrogen and oxygen gas through electrolysis according to the reaction below: 2H2O(D)2H2(g)+O2(g) The resulting hydrogen and oxygen gas mixture produces a total pressure of 210kPa. What is the partial pressure of hydrogen in this gas mixture? 140kPa 50kPa 70kPa 105kPa At extremely high pressures (>>100atm), measured pressures are higher than those predicted by the ideal gas law. Why is this? The volume occupied by the gas molecules cannot be neglected The intermolecular forces acting between the gases cannot be neglected. Both would contribute to higher pressures than those predicted by the ideal gas law. Neither would contribute to higher pressures than predicted by the ideal gas law 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


