Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer both parts and i will upvote; thanks Use the Referonces to access inportant values if needed for this question. Ethanal, C2H6O, is most
please answer both parts and i will upvote; thanks 
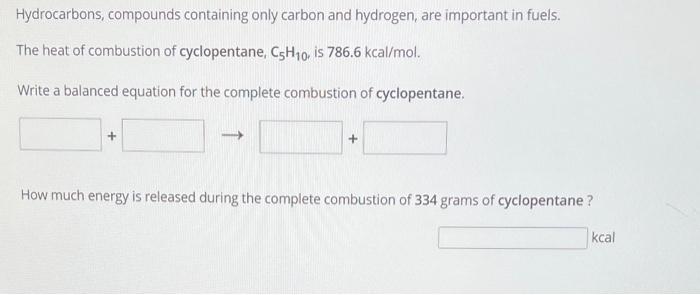
Use the Referonces to access inportant values if needed for this question. Ethanal, C2H6O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuell called gasohol. Ethanolisa renewable ritsourceand ethancl. blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The combustion of one mole of ethanol releases 3267 keal of enersy The combustion of one mole of octane, C8H15, releases 1.308103 kcal of energy. How much energy is relessed during the complete combustion of 356 ursm of octane? Assuming the same efficiency, would 356 grams of ethanol provde more, less, or the same amount of enerby as 256 grams af octane? Bo more G'oup atinmets femain Hydrocarbons, compounds containing only carbon and hydrogen, are important in fuels. The heat of combustion of cyclopentane, C5H10, is 786.6kcal/mol. Write a balanced equation for the complete combustion of cyclopentane. How much energy is released during the complete combustion of 334 grams of cyclopentane? kcal 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


