Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer both parts clearly Fe 2) Using the provided data and excel or another spreadsheet program, fill in the linest output table. Include the
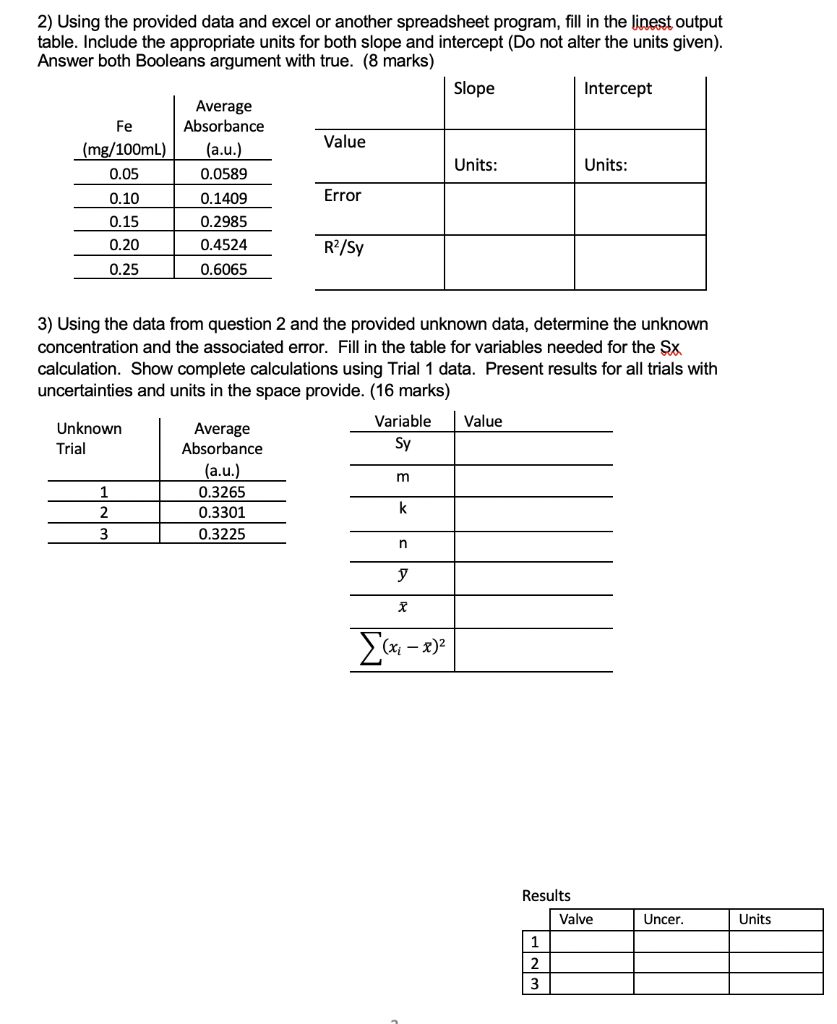
Please answer both parts clearly
Fe 2) Using the provided data and excel or another spreadsheet program, fill in the linest output table. Include the appropriate units for both slope and intercept (Do not alter the units given). Answer both Booleans argument with true. (8 marks) Slope Intercept Average Absorbance Value (mg/100mL) (a.u.) 0.05 Units: Units: 0.0589 0.10 0.1409 Error 0.15 0.2985 0.20 0.4524 0.25 0.6065 R/Sy 3) Using the data from question 2 and the provided unknown data, determine the unknown concentration and the associated error. Fill in the table for variables needed for the SX calculation. Show complete calculations using Trial 1 data. Present results for all trials with uncertainties and units in the space provide. (16 marks) Variable Value Unknown Average Trial Absorbance Sy (a.u.) 1 0.3265 2 0.3301 k 3 0.3225 m n y x {(x 3)2 Results Valve Uncer. Units 1 2 H NM
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


