Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE ANSWER BOTH QUESTIONS!! WILL UPVOTE FOR YOU!! PLS SELECT ALL THAT APPLY !!! Q2) Consider the reaction A+ 2B + C Products The reaction
PLEASE ANSWER BOTH QUESTIONS!! WILL UPVOTE FOR YOU!! PLS
SELECT ALL THAT APPLY !!!
Q2)
Consider the reaction
A+ 2B + C Products
The reaction has the following rate law
rate = k [B]2[C]
If we increase the concentration of A by 2 times, the concentration of B by 3 times and the concentration of C by 5 times, what is the factor does the overall rate increase by?
Group of answer choices
90
30
75
8
15
45
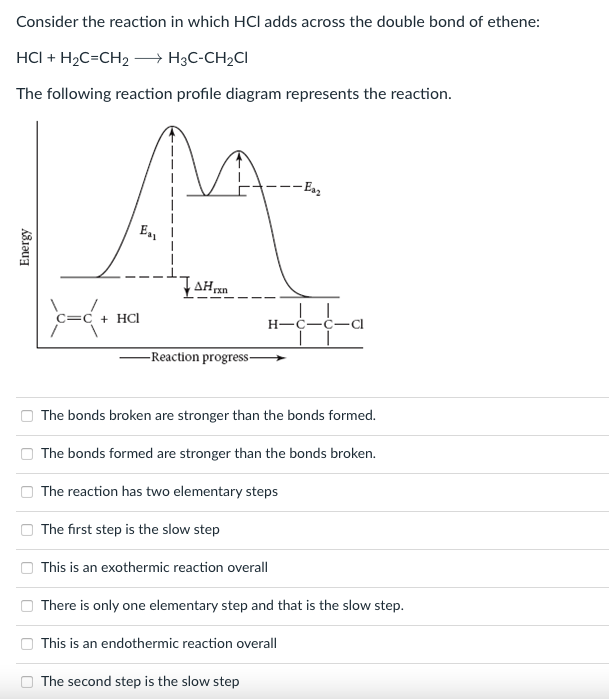
Consider the reaction in which HCl adds across the double bond of ethene: HCI + H2C=CH2 +H3C-CH2C1 The following reaction profile diagram represents the reaction. -Esz M EL Energy TAHUN C=C + HCl 1 - Reaction progress- =c H-C-C-Cl The bonds broken are stronger than the bonds formed. The bonds formed are stronger than the bonds broken. The reaction has two elementary steps The first step is the slow step This is an exothermic reaction overall There is only one elementary step and that is the slow step. This is an endothermic reaction overall The second step is the slow stepStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


