Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer each part in detail! 5. The mass spectrum of BBr2F shown below has an envelope of 6 peaks associated with the molecular ion
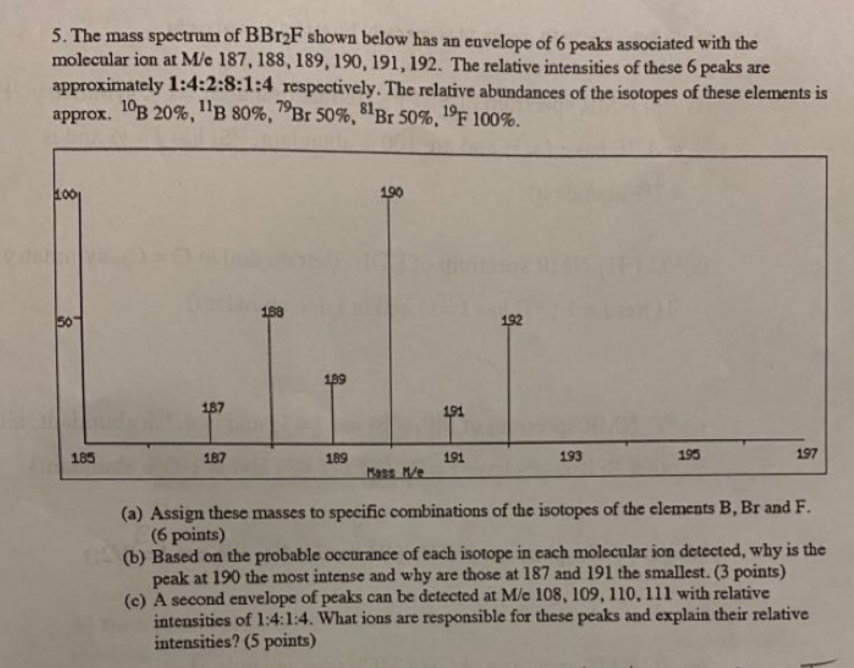
Please answer each part in detail!
5. The mass spectrum of BBr2F shown below has an envelope of 6 peaks associated with the molecular ion at Me 187, 188, 189, 190, 191, 192. The relative intensities of these 6 peaks are approximately 1:4:2:8:1:4 respectively. The relative abundances of the isotopes of these elements is approx. 1B 20%, "B 80%, 79Br 50%, 81Br 50%, 19F 100%. . 1001 190 188 50 192 199 187 191 185 187 189 191 193 195 197 Mass Me (a) Assign these masses to specific combinations of the isotopes of the elements B, Br and F. (6 points) (b) Based on the probable occurance of each isotope in cach molecular ion detected, why is the peak at 190 the most intense and why are those at 187 and 191 the smallest. (3 points) (c) A second envelope of peaks can be detected at Me 108, 109, 110, 111 with relative intensities of 1:4:1:4. What ions are responsible for these peaks and explain their relative intensities? (5 points)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


