Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer every question. NO explanation needed, just the answer is sufficient 11. (4 points) The mechanism of this reaction involves which of these steps
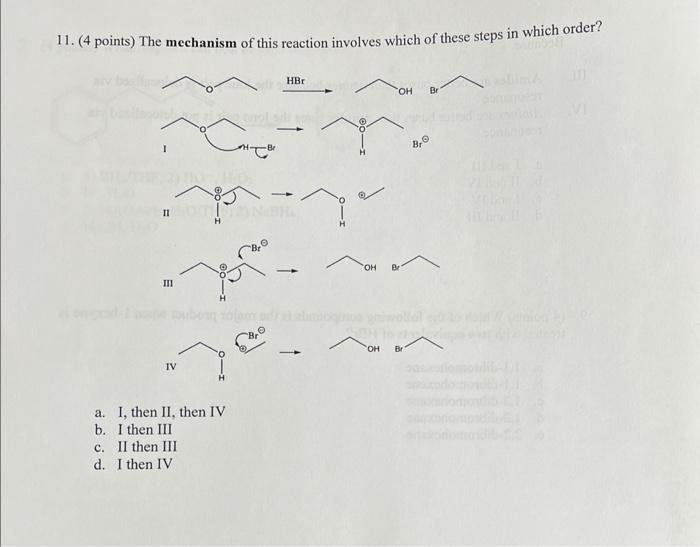
please answer every question. NO explanation needed, just the answer is sufficient
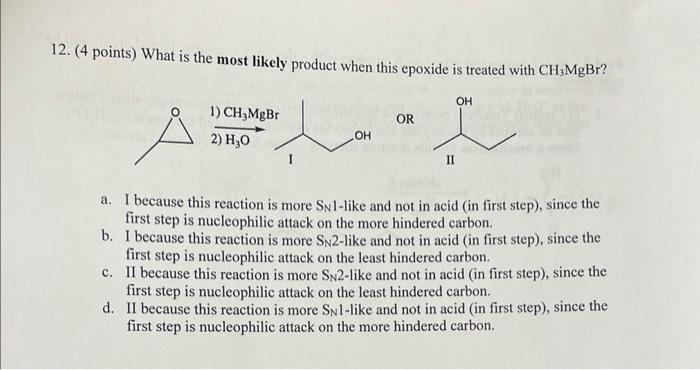
11. (4 points) The mechanism of this reaction involves which of these steps in which order? II () a. I, then II, then IV b. I then III c. II then III d. I then IV 12. (4 points) What is the most likely product when this epoxide is treated with CH3MgBr ? a. I because this reaction is more SN l-like and not in acid (in first step), since the first step is nucleophilic attack on the more hindered carbon. b. I because this reaction is more SN 2-like and not in acid (in first step), since the first step is nucleophilic attack on the least hindered carbon. c. II because this reaction is more SN2-like and not in acid (in first step), since the first step is nucleophilic attack on the least hindered carbon. d. II because this reaction is more SN l-like and not in acid (in first step), since the first step is nucleophilic attack on the more hindered carbon Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


