Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer fully for rating Calcium chloride and sodium phosphate react according to the following balanced chemical equation: 3CaCl2+2Na3PO4Ca3(PO4)2+6NaCl If 507.5g of CaCl2 react with
please answer fully for rating 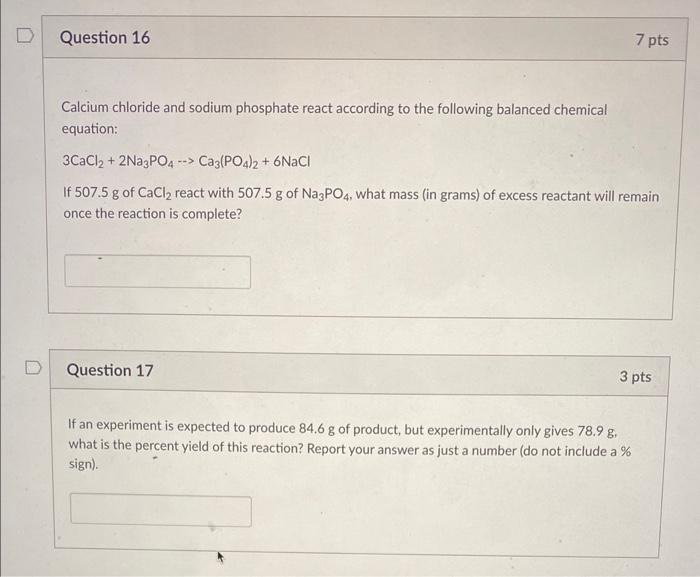
Calcium chloride and sodium phosphate react according to the following balanced chemical equation: 3CaCl2+2Na3PO4Ca3(PO4)2+6NaCl If 507.5g of CaCl2 react with 507.5g of Na3PO4, what mass (in grams) of excess reactant will remain once the reaction is complete? Question 17 3 pts If an experiment is expected to produce 84.6g of product, but experimentally only gives 78.9g, what is the percent yield of this reaction? Report your answer as just a number (do not include a % sign) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


