Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer in detail The determination of any structure of a chemical species begins by examining the electron configurations of the atoms that they are
please answer in detail 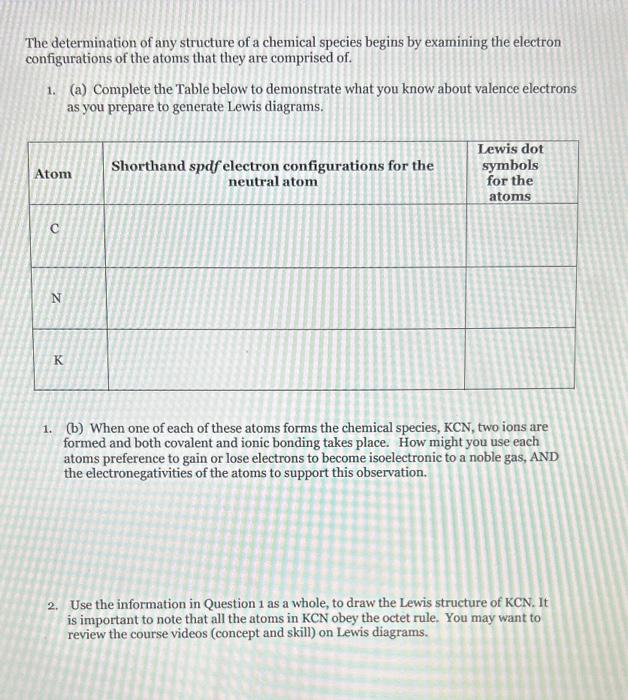

The determination of any structure of a chemical species begins by examining the electron configurations of the atoms that they are comprised of. 1. (a) Complete the Table below to demonstrate what you know about valence electrons as you prepare to generate Lewis diagrams. 1. (b) When one of each of these atoms forms the chemical species, KCN, two ions are formed and both covalent and ionic bonding takes place. How might you use each atoms preference to gain or lose electrons to become isoelectronic to a noble gas, AND the electronegativities of the atoms to support this observation. 2. Use the information in Question 1 as a whole, to draw the Lewis structure of KCN. It is important to note that all the atoms in KCN obey the octet rule. You may want to review the course videos (concept and skill) on Lewis diagrams. (a) A similar chemical species is formed when Ba is in the presence of C and N, Ba(CN) 2. Once again, all atoms obey the octet rule. Explain why you would say the type of bonding does not change. 3. (b) Explain why more C and N atoms are required to form a compound with Ba. It is important to note that all atoms obey the octet rule. Hint generate the Lewis diagram for Ba(CN)2 to help you answer this 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


