Answered step by step
Verified Expert Solution
Question
1 Approved Answer
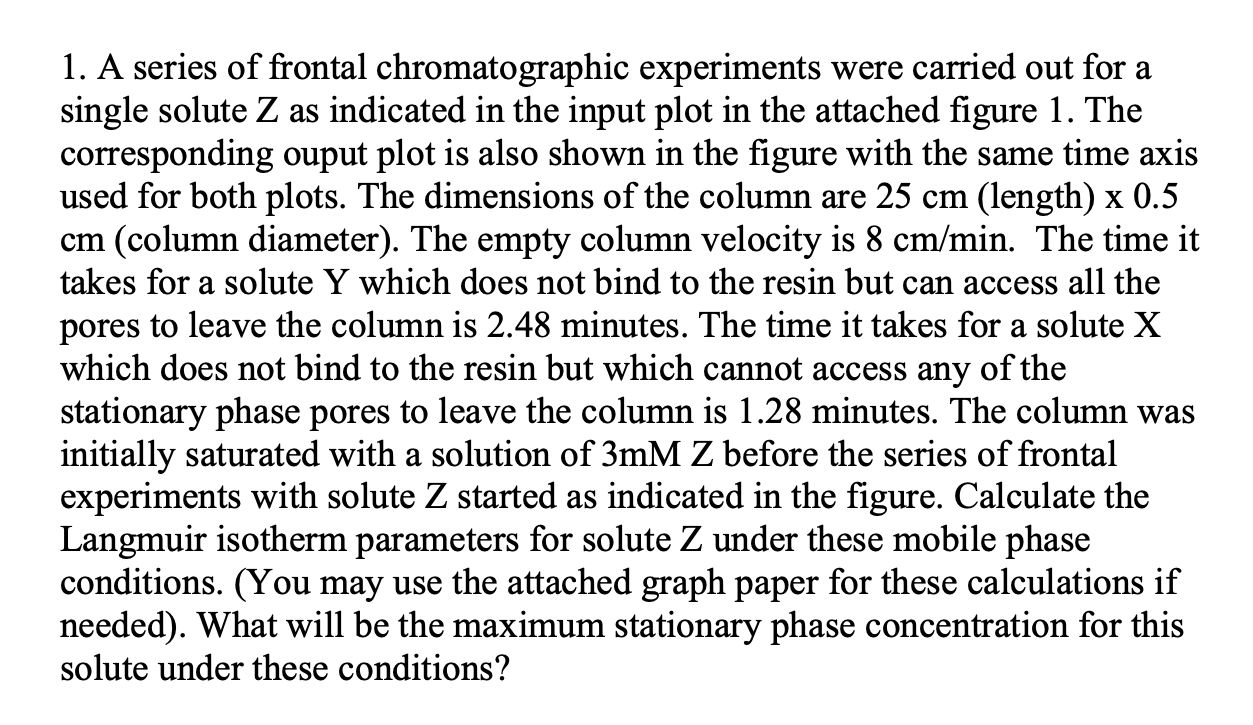
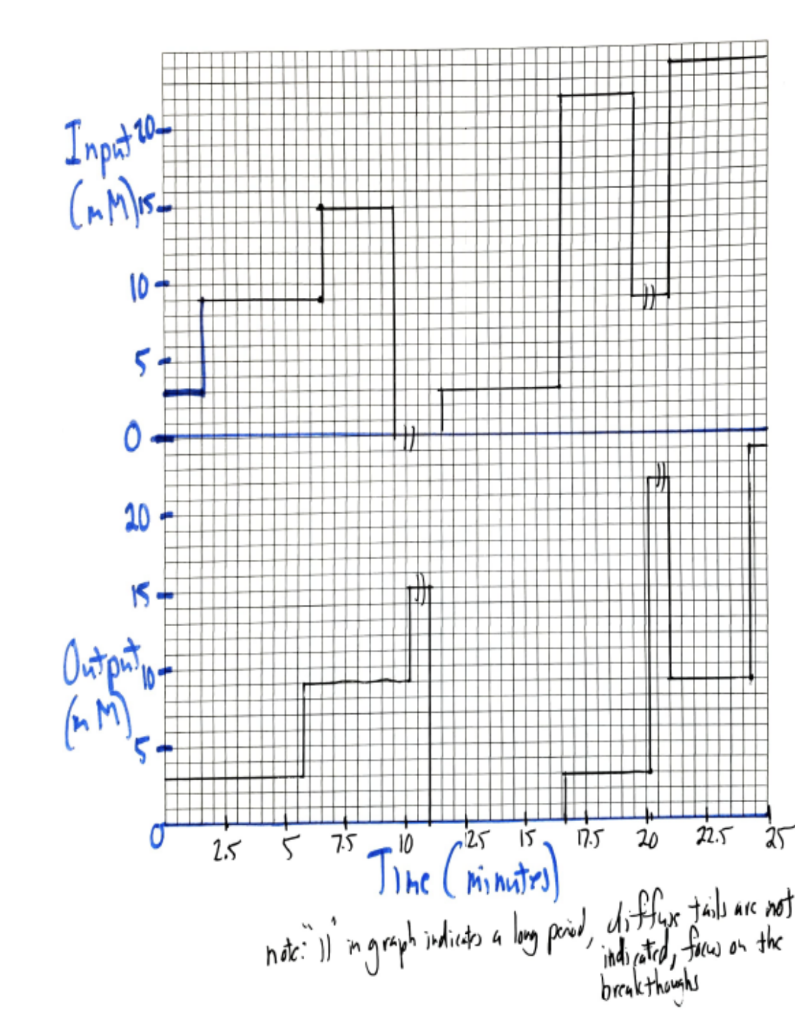
Please answer is detail and step to step format. 1. A series of frontal chromatographic experiments were carried out for a single solute Z as
Please answer is detail and step to step format.
1. A series of frontal chromatographic experiments were carried out for a single solute Z as indicated in the input plot in the attached figure 1 . The corresponding ouput plot is also shown in the figure with the same time axis used for both plots. The dimensions of the column are 25cm (length) 0.5 cm (column diameter). The empty column velocity is 8cm/min. The time it takes for a solute Y which does not bind to the resin but can access all the pores to leave the column is 2.48 minutes. The time it takes for a solute X which does not bind to the resin but which cannot access any of the stationary phase pores to leave the column is 1.28 minutes. The column was initially saturated with a solution of 3mMZ before the series of frontal experiments with solute Z started as indicated in the figure. Calculate the Langmuir isotherm parameters for solute Z under these mobile phase conditions. (You may use the attached graph paper for these calculations if needed). What will be the maximum stationary phase concentration for this solute under these conditionsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


