Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer it all this is my last post question thank you so much A. Determine the empirical formula for the following situations. Show all
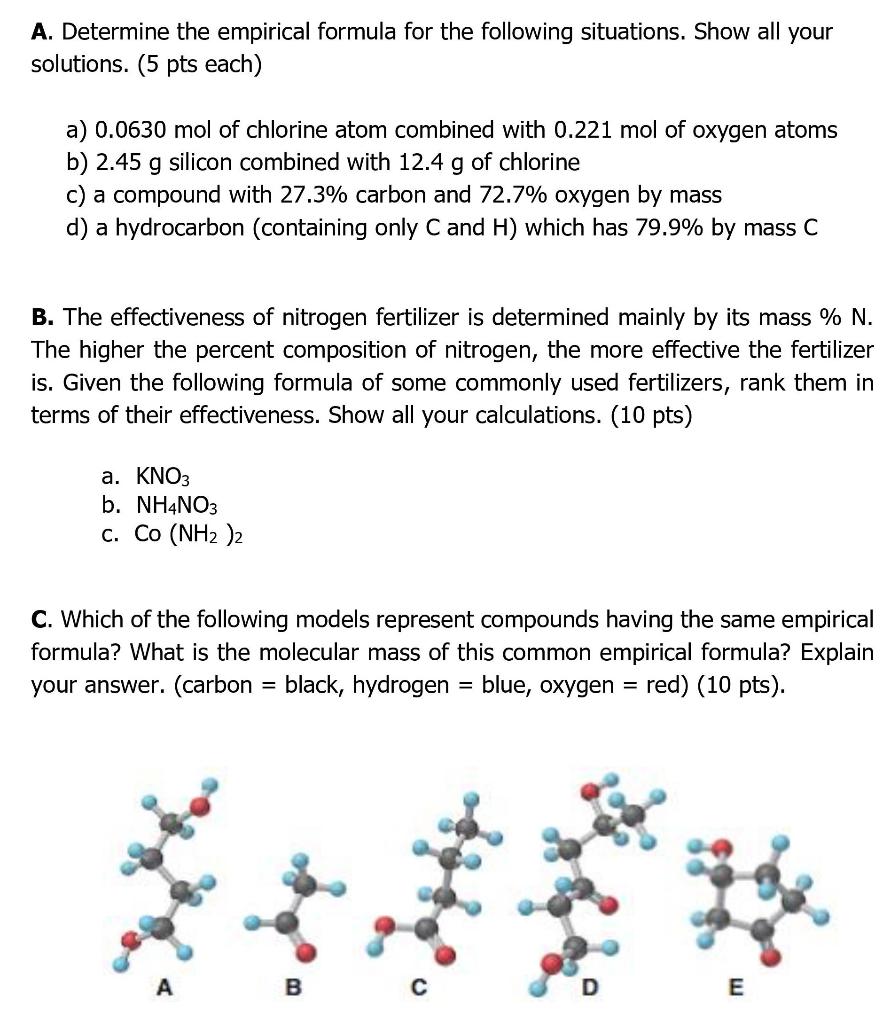
Please answer it all this is my last post question thank you so much
A. Determine the empirical formula for the following situations. Show all your solutions. (5 pts each) a) 0.0630 mol of chlorine atom combined with 0.221 mol of oxygen atoms b) 2.45 g silicon combined with 12.4 g of chlorine c) a compound with 27.3% carbon and 72.7% oxygen by mass d) a hydrocarbon (containing only C and H) which has 79.9% by mass C B. The effectiveness of nitrogen fertilizer is determined mainly by its mass % N. The higher the percent composition of nitrogen, the more effective the fertilizer is. Given the following formula of some commonly used fertilizers, rank them in terms of their effectiveness. Show all your calculations. (10 pts) a. KNO3 b. NH4NO3 C. CO (NH2)2 C. Which of the following models represent compounds having the same empirical formula? What is the molecular mass of this common empirical formula? Explain your answer. (carbon black, hydrogen blue, oxygen = red) (10 pts). = =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


