Answered step by step
Verified Expert Solution
Question
1 Approved Answer
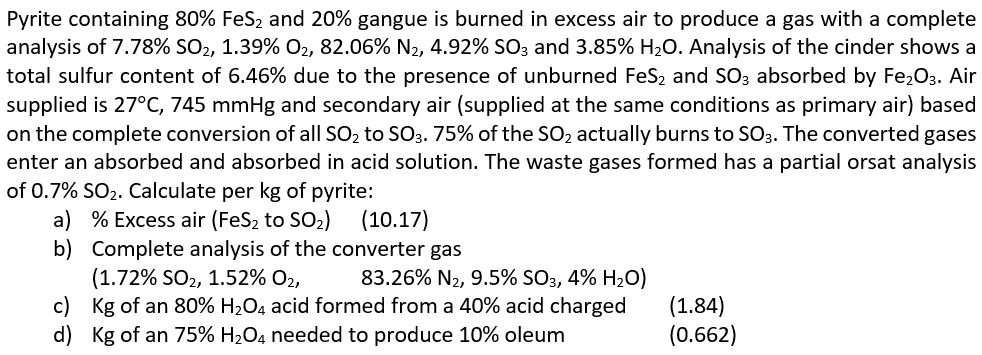
Please answer items c and d. Pyrite containing 80% FeS2 and 20% gangue is burned in excess air to produce a gas with a complete
Please answer items c and d.
Pyrite containing 80% FeS2 and 20% gangue is burned in excess air to produce a gas with a complete analysis of 7.78% SO2, 1.39% O2, 82.06% N2, 4.92% SO3 and 3.85% H20. Analysis of the cinder shows a total sulfur content of 6.46% due to the presence of unburned FeS2 and SO3 absorbed by Fe2O3. Air supplied is 27C, 745 mmHg and secondary air (supplied at the same conditions as primary air) based on the complete conversion of all SO2 to SO3.75% of the SO2 actually burns to SO3. The converted gases enter an absorbed and absorbed in acid solution. The waste gases formed has a partial orsat analysis of 0.7% SO2. Calculate per kg of pyrite: a) % Excess air (FeS2 to SO2) (10.17) b) Complete analysis of the converter gas (1.72% SO2, 1.52% O2, 83.26% N2, 9.5% SO3, 4% H2O) c) Kg of an 80% H2O4 acid formed from a 40% acid charged (1.84) d) Kg of an 75% H2O4 needed to produce 10% oleum (0.662)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


