please answer just N**, N(N+2) and Effective x(g/mol)
Everything was posted that's the question already
this is everything
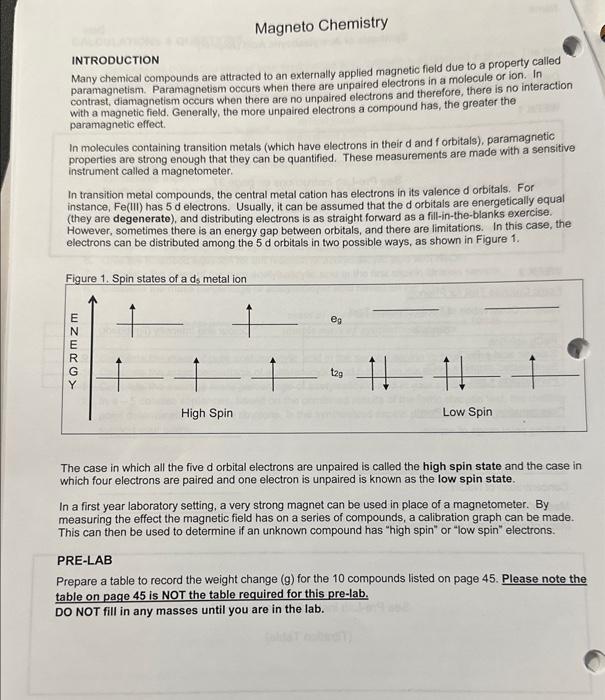
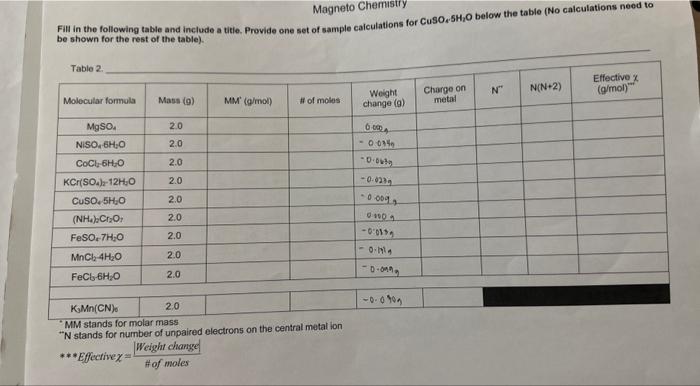
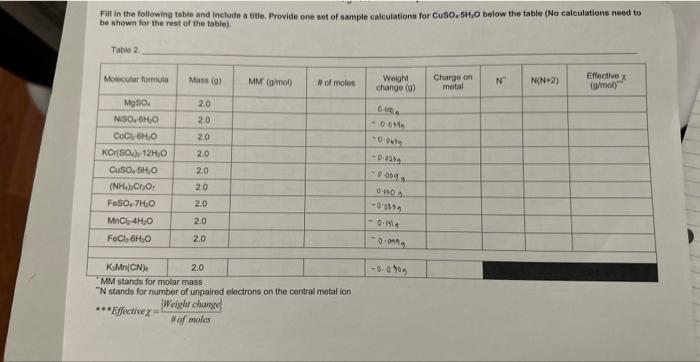
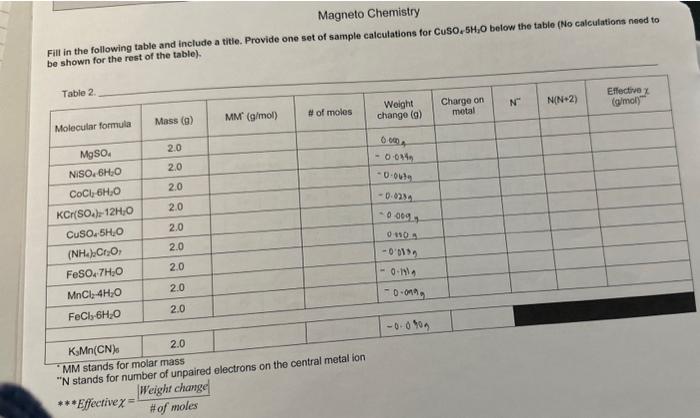
Magneto Chemistry Fill in the following table and include a titie. Provide one set of sample calculations for CuSO2SH2O below the table (No calculations need to be shown for the rest of the table). \begin{tabular}{|r|r|r|c|} \hline KMn(CN) & 2.0 \\ \hline \end{tabular} "MM stands for molar mass " N stands for number of unpaired electrons on the central metal ion Effective =HofmolesWeightchange Fill in the following table and include a titie. Provide one set of aample calcuiations for CuSO, 5CjO below the table (No calculations need to be shown for the rast of the table). Table 2 NM stands for melar mass N stands for number of unpaired ekuctrons on the contral metal ion Print and fill in the table found on the last page, Show sample calculations for CuSO45H2O below the table for the last 4 columns (charge on metal, N,N(N+2), effective x ). Hand in this page with your report along with Questions 1-6 and your graph. 1. Prepare a calibration graph with N(N+2) on the x-axis and the effective on the y-axis, using all of your points EXCEPT the KMMn(CN). Draw the line of best fit through all the points. 2. a) For KMn(CN) what is the charge on the Mn? b) How many unpaired electron will it have if it is high spin (ie., the energy gap between the d-orbitals is small)? c) How many if it is low spin (i.e, the energy gap between the d-orbitals is large)? 3. Calculate the effective for KMMn(CN)s. 4. Use the graph to calculate N(N+2) value by interpolation for KMnn(CN). 5. You now have a quadratic equation for the number of unpaired electrons (N) in K3Mn(CN). To make the solution simple just plug your number of N(N+2) from the graph into the equation below (your value should be positive). N=22+4+(4valuefromgraph) 6. Does the value you calculated for N in the above question indicate that KMn(CN) is high spin or low spin? INTRODUCTION Many chemical compounds are attracted to an externally applied magnetic field due to a property called paramagnetism. Paramagnetism occurs when there are unpaired electrons in a molecule or ion. In contrast, diamagnetism occurs when there are no unpaired electrons and therefore, there is no interaction with a magnetic field. Generally, the more unpaired electrons a compound has, the greater the paramagnetic effect. In molecules containing transition metals (which have electrons in their d and f orbitals), paramagnetic properties are strong enough that they can be quantified. These measurements are made with a sensitive instrument called a magnetometer. In transition metal compounds, the central metal cation has electrons in its valence d orbitals. For instance, Fe(IIi) has 5 d electrons. Usually, it can be assumed that the d orbitals are energetically equal (they are degenerate), and distributing electrons is as straight forward as a fill-in-the-blanks exercise. However, sometimes there is an energy gap between orbitals, and there are limitations. In this case, the electrons can be distributed among the 5d orbitals in two possible ways, as shown in Figure 1. The case in which all the five d orbital electrons are unpaired is called the high spin state and the case in which four electrons are paired and one electron is unpaired is known as the low spin state. In a first year laboratory setting, a very strong magnet can be used in place of a magnetometer. By measuring the effect the magnetic field has on a series of compounds, a calibration graph can be made. This can then be used to determine if an unknown compound has "high spin" or "low spin" electrons. PRE-LAB Prepare a table to record the weight change (g) for the 10 compounds listed on page 45 . Please note the table on page 45 is NOT the table required for this pre-lab. DO NOT fill in any masses until you are in the lab. QURE GEFORE YOUBEGIN: - Work in partners. Take turns weighing the samples. - Record exactly what is displayed on the balance into your data. This includes all decimal places and signs (+/-). - DO NOT move the balances. The main setup has already been done and looks like the diagram below. Please do not change anything about the setup when doing the experiment. 1. Make sure the setup is correct and that the balance is zeroed or tared. 2. Take your first sample and carefully place it in the hole over the magnets. A rubber collar aroune the test tube will set it to the correct height above the magnets. DO NOT move the collar. 3. Take the balance reading, including the sign and the decimal places, and record it in your data. 4. Repeat the above procedure for each of the other samples. Between trials when there is no san above the magnets, make sure the balance reading is 0 . If not, re-zero the balance before takir the next measurement. 5. After all of the samples are measured, place everything back where you found it. CALCULATIONS \& QUESTIONS Print and fill in the table found on the last page. Show sample calculations for CuSO45H2O below the table for the last 4 columns (charge on metal, N,N(N+2 ), effective x ). Hand in this page with your report along with Questions 1-6 and your graph. 1. Prepare a calibration graph with N(N+2) on the x-axis and the effective x on the y-axis, using all of y points EXCEPT the KPMn(CN). Draw the line of best fit through all the points. 2. a) For KMMn(CN) shat is the charge on the Mn? b) How many unpaired electron will it have if it is high spin (i.e., the energy gap between the d-orbital: is small)? c) How many if it is low spin (i.e., the energy gap between the d-orbitals is large)? 3. Calculate the effective x for KMn(CN). 4. Use the graph to calculate N(N+2) value by interpolation for K.Mn(CN)s. 5. You now have a quadratic equation for the number of unpaired electrons (N) in KJMn(CN). To make th solution simple just plug your number of N(N+2) from the graph into the equation below (your value should be positive), N=22+4+(4valuefromgraph) 6. Does the value you calculated for N in the above question indicate that K3Mn(CN) is high spin or low spin? Magneto Chemistry Fill in the following table and include a titie. Provide one set of sample calculations for CuSO, 5SH4O below the table (No calculations need to ha shown for the rest of the table). \begin{tabular}{l|l} KsMn(CN)0 & 2.0 \end{tabular} - MM stands for molar mass " N stands for number of unpaired electrons on the central metal ion ***Effective =#ofmolesWeightchange|