Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer Q5 I have attached all previous questions to be able to do so 1. Deperimentally Determining KC at 3.8C. A student loads 3.0mL
please answer Q5 I have attached all previous questions to be able to do so
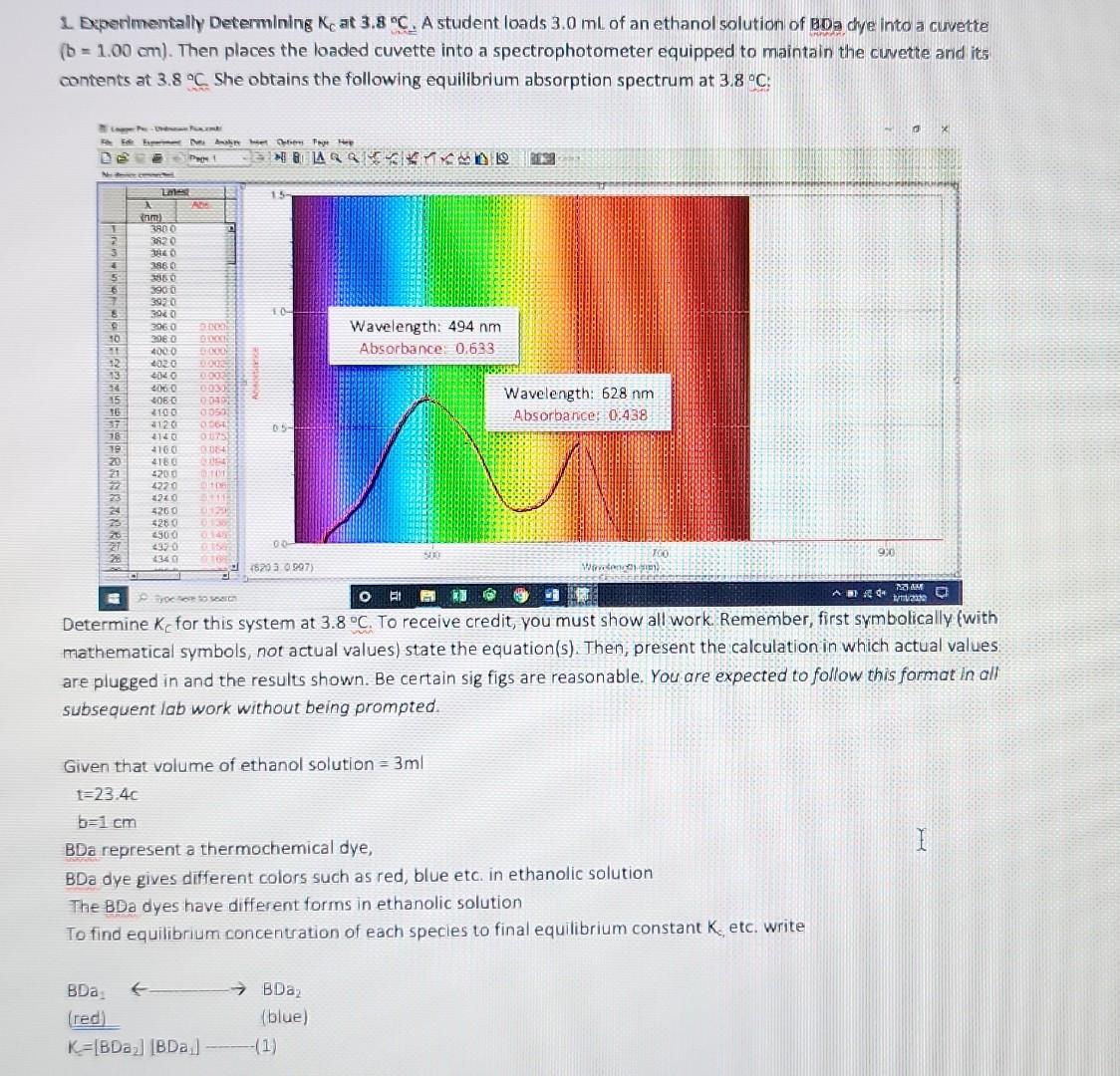
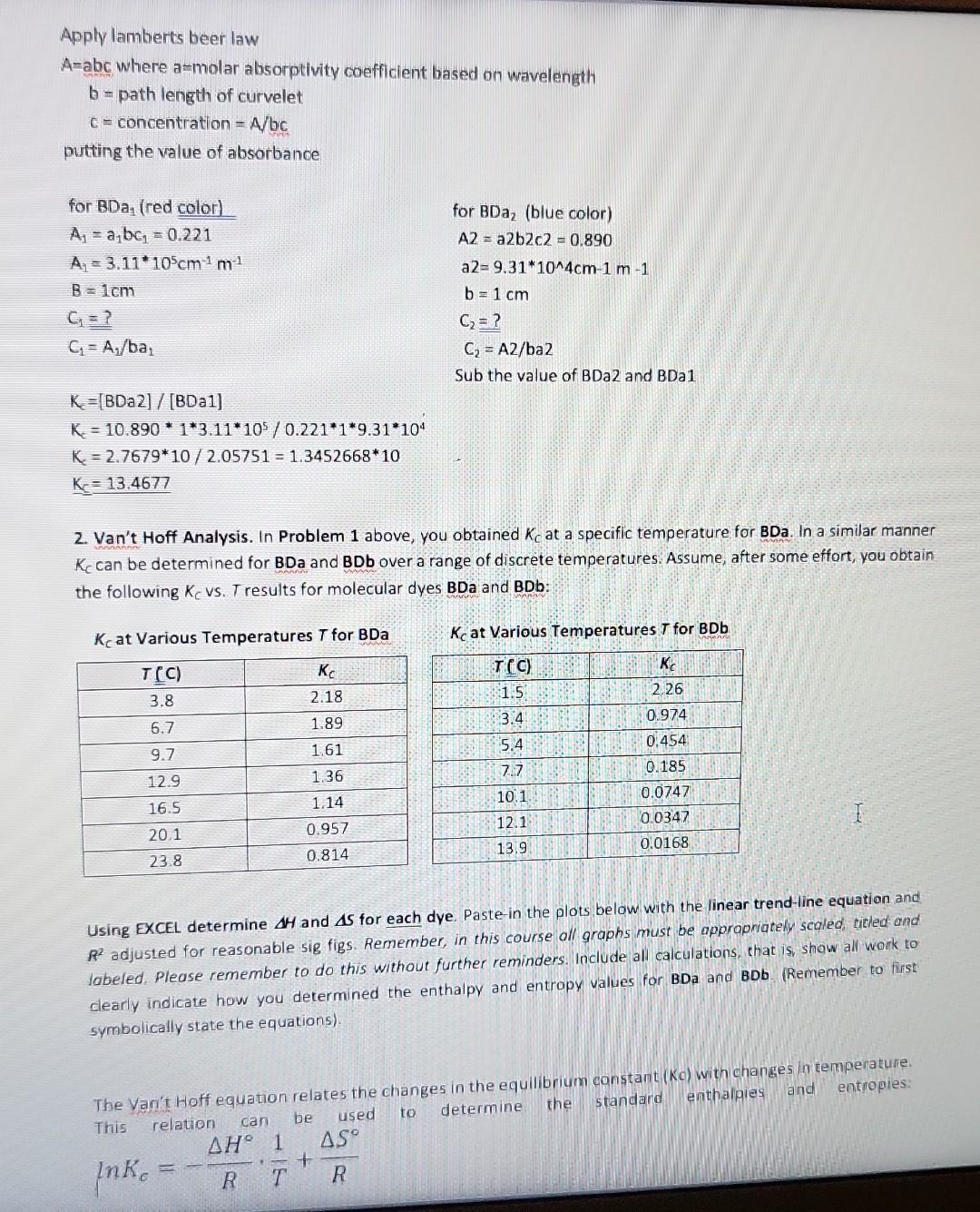
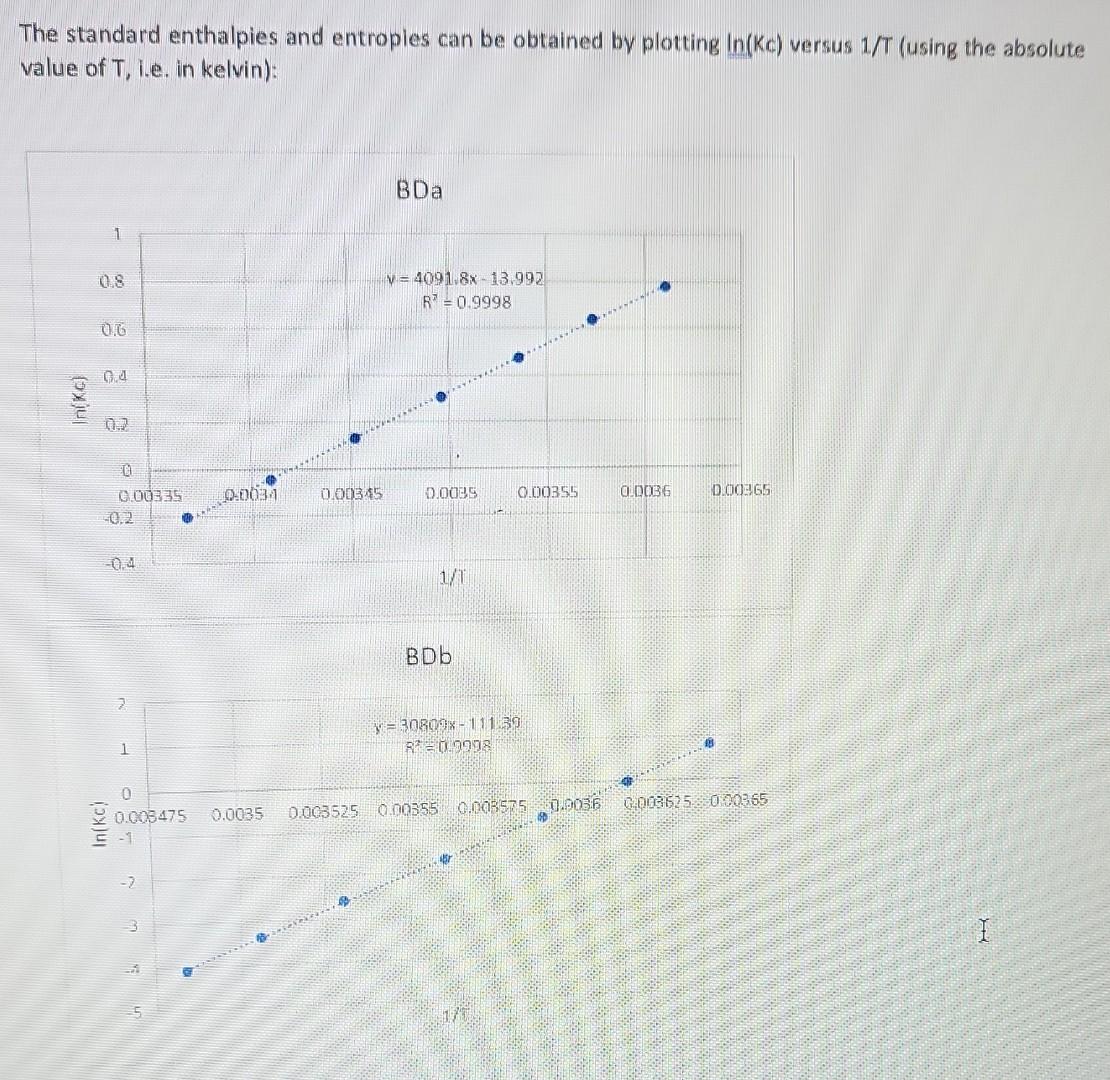
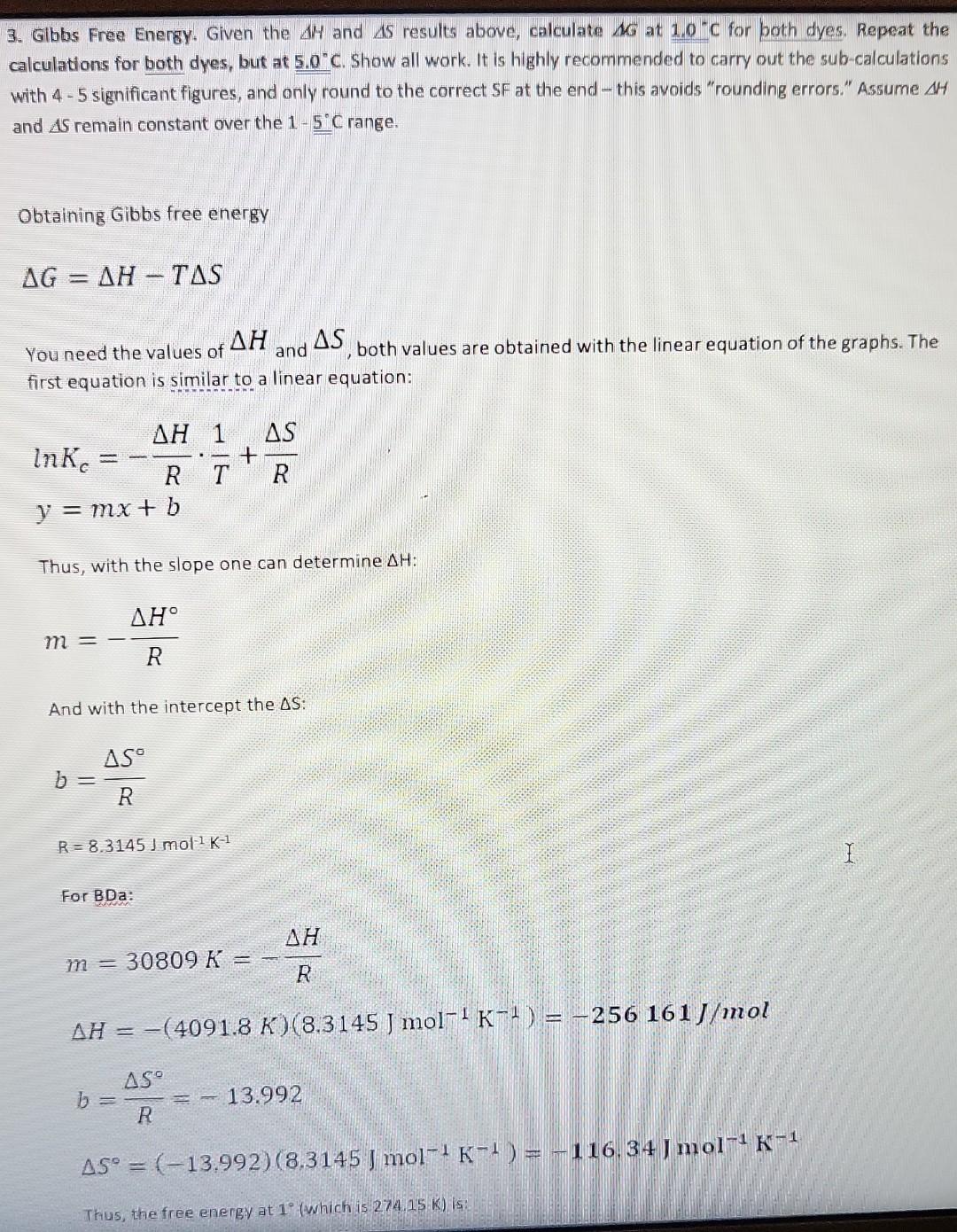

1. Deperimentally Determining KC at 3.8C. A student loads 3.0mL of an ethanol solution of BDa dye into a curvette (b=1.00cm). Then places the loaded cuvette into a spectrophotometer equipped to maintain the cuvette arid its contents at 3.8C. She obtains the following equilibrium absorption spectrum at 3.8C : Determine KC for this system at 3.8C. To receive credit, you must show all work Remember, first symbolically fwith mathematical symbols, not actual values) state the equation(s). Then, present the calculation in which actual values: are plugged in and the results shown. Be certain sig figs are reasonable. You are expected to follow this format in all subsequent lab work without being prompted. Given that volume of ethanol solution =3ml t=23.4cb=1cm BDa represent a thermochemical dye, BDa dye gives different colors such as red, blue etc. in ethanolic solution The BDa dyes have different forms in ethanolic solution To find equilibrium concentration of each specles to final equilibrium constant K, etc. write BDa1BDa2(red)K=[BDBa2][BDa1](1) b= path length of curvelet c= concentration =A/bc putting the value of absorbance forBDa1(redcolor)A1=a1b1=0.221A1=3.11105cm1m1B=1cmC1=?C1=A1/ba1forBDa(bluecolor)A2=a2b2c2=0.890a2=9.31104cm1m1B=1cmC2=?C2=A2/ba2 Kc=[BDa2]/[BDa1] KK=10.89013.11105/0.22119.31104K=2.767910/2.05751=1.345266810K=13.4677 Sub the value of BDa2 and BDa1 2. Van't Hoff Analysis. In Problem 1 above, you obtained Kc at a specific temperature for BDa. In a similar manner KC can be determined for BDa and BDb over a range of discrete temperatures. Assume, after some effort, you obtain the following Kc vs. T results for molecular dyes BDa and BDb: KC at Various Temperatures T for BDa KC at Various Temperatures T for BDb Using EXCEL determine H and S for each dye. Paste-in the plots below with the linear trend-line equation and R2 adjusted for reasonable sig figs. Remember, in this course all graphs must be apprapriately sealed, eitled and labeled. Please remember to do this without further reminders. Include all calculations, that is, show all work to clearly indicate how you determined the enthalpy and entropy values for BDa and BOb. (Remember to first symbolically state the equations). The Van't Hoff equation relates the changes in the equilibrium canstant ( Kc) with changes in temperature. This relation can be used to determine the standard enthalgies and entropies: lnKc=RHT1+RS The standard enthalpies and entroples can be obtained by plotting ln(Kc) versus 1/T (using the absolute value of T, i.e. in kelvin): 3. Glbbs Free Energy. Given the H and S results above, calculate G at 1.0C for poth dyes. Repeat the calculations for both dyes, but at 5.0C. Show all work. It is highly recommended to carry out the sub-calculations with 4 - 5 significant figures, and only round to the correct SF at the end - this avoids "rounding errors." Assume H and S remain constant over the 15C range. Obtaining Gibbs free energy G=HTS You need the values of H and S, both values are obtained with the linear equation of the graphs. The first equation is similar to a linear equation: lnKc=RHT1+RSy=mx+b Thus, with the slope one can determine H : m=RH And with the intercept the S : b=RSR=8.3145/mol1K1 For BDa: m=30809K=RHH=(4091.8K)(8.3145Jmol1K1)=256161J/molb=RS=13.992S=(13.992)(8.3145Jmol1K1)=116.34Jmol Thus, the free energy at 1 (which is 274.15 K. ) is: G=HTSG=34021molJ+(274.15K)(116.34Jmol1K1)=2126J/mol The free energ y at 5 (which is 278.15K ) is: G=34021molJ+(278.15K)(116.34Jmol1K1)=1661J/mol For BDb: m=30809K=RHH=(30809K)(8.3145Jmol1K1)=256161J/molb=RS=111.39S=(111.39)(8.3145Jmol1K1)=922.9Jmol1K1 The free energy at 1 (which is 274.15K ) is: G=256161molJ+(274.15K)(922.9Jmol1K1)=3148J/mol The free energy at 5 (which is 278.15K ) is: G=256161molJ+(278.15K)(922.9Jmol1K1)=543.6J/mol 4. Analysis I. Given the results above, what do the calculated G results indicate about the directionality (product v5. reactant) of each system (BDa and BDb) at 5.0C versus 1.0C ? G, for both reactions, increases as the temperature increases. This means that less product will form at higher temperatures. 5. Analysis II. As you know, certain raw vegetables when subjected to freezing temperatures loose desirable qualities such as flavor and firmness. Suppose you are tasked with designing an inexpensive thermochromic thermometer that can be attached to vegetable shipping pallets and indicate if freezing temperatures exist. In this application the thermometer must sharply undergo a chromic shift (give a rapid, distinct color change) around 24C. Given the results from Questions 2 - 4 above, and assuming the E and x+x values of both dyes are essentially identical, which dye, BDa or BDb, would be the best choice for such a thermochromic thermometer? Clearly justify your claim using the relevant data. To receive credit, you must respond with well written, very legible, complete sentences that correctly employs chemical terminology and concepts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


