Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE ANSWER SOON (be clear and be sure to include proper units in answer) Use data from the thermodynamic tables and neglect temperature dependence of
PLEASE ANSWER SOON (be clear and be sure to include proper units in answer) 
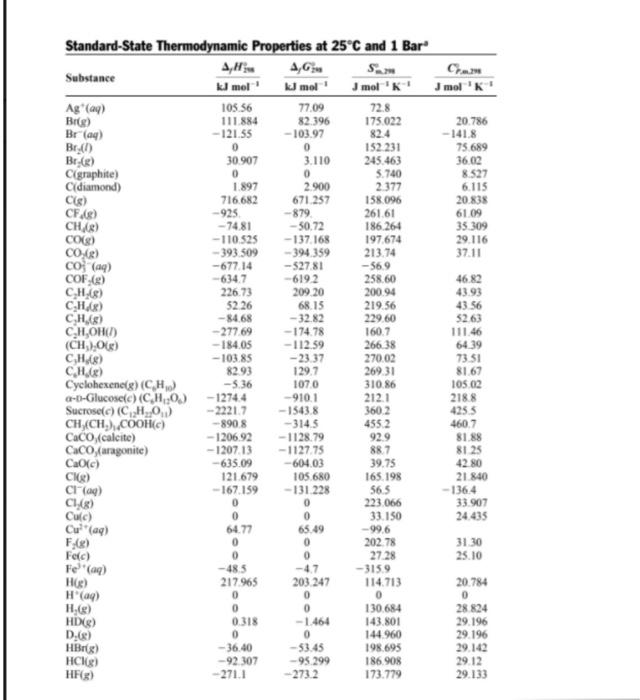
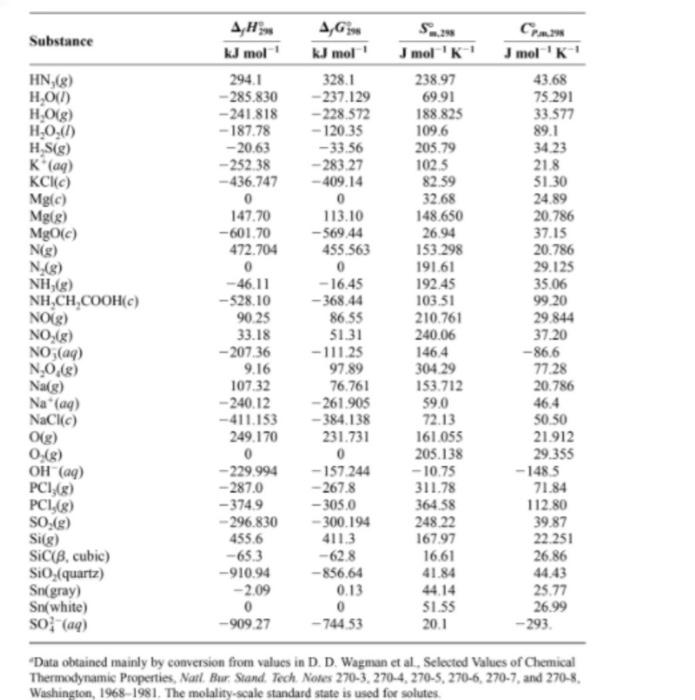
Use data from the thermodynamic tables and neglect temperature dependence of Acp to find AH 700 for: 2HN3 (9) + 2NO (9) H202 (1) + 4N2 (9) Jmol 'K' 77.09 0 CH/8) Standard-State Thermodynamic Properties at 25C and 1 Bar A,G Substance kl mol' kJ mol mol 'K! Ag (ay) 105.56 72.8 Bilge) 111.884 82.396 175022 Bray) -121.55 -103.97 82.4 Br.) 0 0 152 231 Br(e) 30.907 3.110 245.463 Cigraphite) 0 5.740 Cldiamond) 1.897 2.900 2.377 Cs) 716682 671.257 158.096 CF18) -925 -879 261.61 - 74.81 --50.72 186.264 CO(g) -110.525 -137.168 197.674 CO (2) -393.509 -394359 213.74 co; (aq) -677.14 -52781 -569 COF.(e) -6347 -6192 258.60 CH,8) 226.73 209 20 200.94 CH.) 52.26 68.15 219.56 CHAS) - 84.68 - 3282 229.60 CH,OHCI) -277.69 -17478 160.7 (CH, O) -184.05 - 112.59 266.38 CH,8) -103.85 -2337 270.02 CH.) 82.93 129.7 26931 Cyclohexenelg) (CH) -5.36 1070 310.86 a-t-Glucosete) (CH20.) -12744 -910.1 212.1 Sucrose) (H20) -22217 - 15438 360.2 CH (CH),COOH) -8908 - 314.5 4552 CaCO(calcite) -1206.92 -1128.79 92.9 Caco (aragonite) -120713 -112775 88.7 Ca(c) - 635.09 -604.03 39.75 ) 121.679 105.680 165.198 Cl(aq) - 167.159 - 131 228 56.5 C1,[8) 0 0 223.066 Cuc) 0 0 33.150 Cu(aq) 64.77 65.49 -99.6 0 0 202.78 Ferc) 0 27.28 -48.5 -47 -3159 H) 217965 203.247 114713 H(a) 0 0 0 H.) 0 0 130.684 HD) 0.318 -1.464 143.801 D:68) 0 144 960 HBrg) - 36.40 -53.45 198.695 HCR) -92 307 -95.299 186.908 HF) 2711 2732 173.779 20.786 - 141.8 75.689 36,02 8.527 6.115 20.838 61 09 35309 29.116 37.11 46.82 43.93 43.56 52.63 111.46 64.39 73.51 81.67 105.02 2188 4255 460.7 81.88 8125 42.80 21.840 -136.4 33.907 24.435 FAR) Fe (aq) 31.30 25.10 20.784 0 28 824 29.196 29.196 29.142 29.12 29.133 0 Substance HN (8) H.0(1) H.O(g) H.0.(1) H () K(aq) KClic) Mg(c) Mgle) MgO(c) Ng) N. (8) NH) NH,CH,COOH(c) NO(g) NO (8) NO3(aq) N,0.6) Nais) Na'(a) NaCK(C) O(g) 0.08) OH(aq) PC1,8) PC1,8) sorg) Sig) SiCIB, cubic) Sio, (quartz) Sn(gray) Sn(white) so (aq) AH KJ mol 294.1 -285.830 - 241.818 -187.78 -20.63 - 252.38 -436.747 0 147.70 -601.70 472.704 0 -46.11 -528.10 90.25 33.18 -207.36 9.16 107.32 - 240.12 -411.153 249.170 0 - 229.994 -287,0 -3749 -- 296.830 455.6 -65.3 -910.94 -2.09 0 -909.27 4,6 kJ mol' 328.1 -237.129 -228.572 - 120.35 -33.56 -283.27 -409.14 0 113.10 --569.44 455563 0 -16.45 -368.44 86.55 51.31 -111.25 97.89 76.761 - 261.905 -384.138 231.731 0 -157.244 -267.8 -305.0 -300.194 411.3 -628 -856.64 0.13 0 -744 53 Jmol 238 97 6991 188.825 109.6 205.79 102.5 82.59 32.68 148.650 26.94 153.298 191.61 192.45 103.51 210.761 240.06 146.4 304 29 153.712 Jmol 'K' 43.68 75.291 33.577 89.1 34.23 21.8 51.30 24.89 20.786 37.15 20.786 29.125 35.06 99.20 29 844 37.20 -86.6 77.28 20.786 46.4 50.50 21.912 29.355 -1485 71.84 112.80 39.87 22.251 26.86 44.43 25.77 26.99 - 293 59,0 72.13 161.055 205.138 -10.75 311.78 364.58 248.22 167.97 16.61 41.84 51.55 20.1 Data obtained mainly by conversion from values in D D. Wagman et al. Selected Values of Chemical Thermodynamic Properties, Natl. Bur Stand. Tech. Notes 270-3.270-4, 270-5, 270-6, 270-7 and 270-8 Washington, 1968-1981. The molality-scale standard state is used for solutes 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


