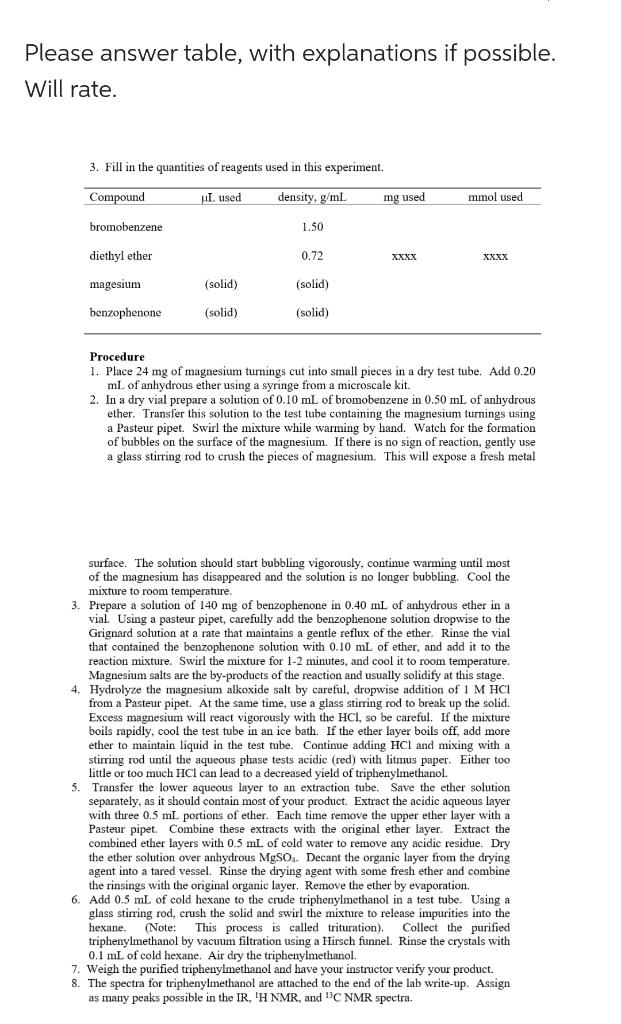
Please answer table, with explanations if possible. Will rate. 3. Fill in the quantities of reagents used in this experiment. Procedure 1. Place 24mg of magnesium turnings cut into small pieces in a dry test tube. Add 0.20 mL of anhydrous ether using a syringe from a microscale kit. 2. In a dry vial prepare a solution of 0.10mL of bromobenzene in 0.50mL of anhydrous ether. Transfer this solution to the test tube containing the magnesium turnings using a Pasteur pipet. Swirl the mixture while warming by hand. Watch for the formation of bubbles on the surface of the magnesium. If there is no sign of reaction, gently use a glass stirring rod to crush the pieces of magnesium. This will expose a fresh metal surface. The solution should start bubbling vigorously, continue warming until most of the magnesium has disappeared and the solution is no longer bubbling. Cool the mixture to room temperature. 3. Prepare a solution of 140mg of benzophenone in 0.40mL of anhydrous ether in a vial. Using a pasteur pipet, carefully add the benzophenone solution dropwise to the Grignard solution at a rate that maintains a gentle reflux of the ether. Rinse the vial that contained the benzophenone solution with 0.10mL of ether, and add it to the reaction mixture. Swirl the mixture for 12 minutes, and cool it to room temperature. Magnesium salts are the by-products of the reaction and usually solidify at this stage. 4. Hydrolyze the magnesium alkoxide salt by careful, dropwise addition of 1MHCl from a Pasteur pipet. At the same time, use a glass stirring rod to break up the solid. Excess magnesium will react vigorously with the HCl, so be careful. If the mixture boils rapidly, cool the test tube in an ice bath. If the ether layer boils off, add more ether to maintain liquid in the test tube. Continue adding HCl and mixing with a stirring rod until the aqueous phase tests acidic (red) with litmus paper. Either too little or too much HCl can lead to a decreased yield of triphenylmethanol. 5. Transfer the lower aqueous layer to an extraction tube. Save the ether solution separately, as it should contain most of your product. Extract the acidic aqueous layer with three 0.5mL portions of ether. Each time remove the upper ether layer with a Pasteur pipet. Combine these extracts with the original ether layer. Extract the combined ether layers with 0.5mL of cold water to remove any acidic residue. Dry the ether solution over anhydrous MgSO4. Decant the organic layer from the drying agent into a tared vessel. Rinse the drying agent with some fresh ether and combine the rinsings with the original organic layer. Remove the ether by evaporation. 6. Add 0.5mL of cold hexane to the crude triphenylmethanol in a test tube. Using a glass stirring rod, crush the solid and swirl the mixture to release impurities into the hexane. (Note: This process is called trituration). Collect the purified triphenylmethanol by vacuum filtration using a Hirsch funnel. Rinse the crystals with 0.1mL of cold hexane. Air dry the triphenylmethanol. 7. Weigh the purified triphenylmethanol and have your instructor verify your product. 8. The spectra for triphenylmethanol are attached to the end of the lab write-up. Assign as many peaks possible in the IR, 1HNMR, and 13C NMR spectra







