Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer the above question (all parts) One mole of gas, at temperature, T=T0 is isothermally compressed from pressure, P=P1 to pressure, P=P2. The gas
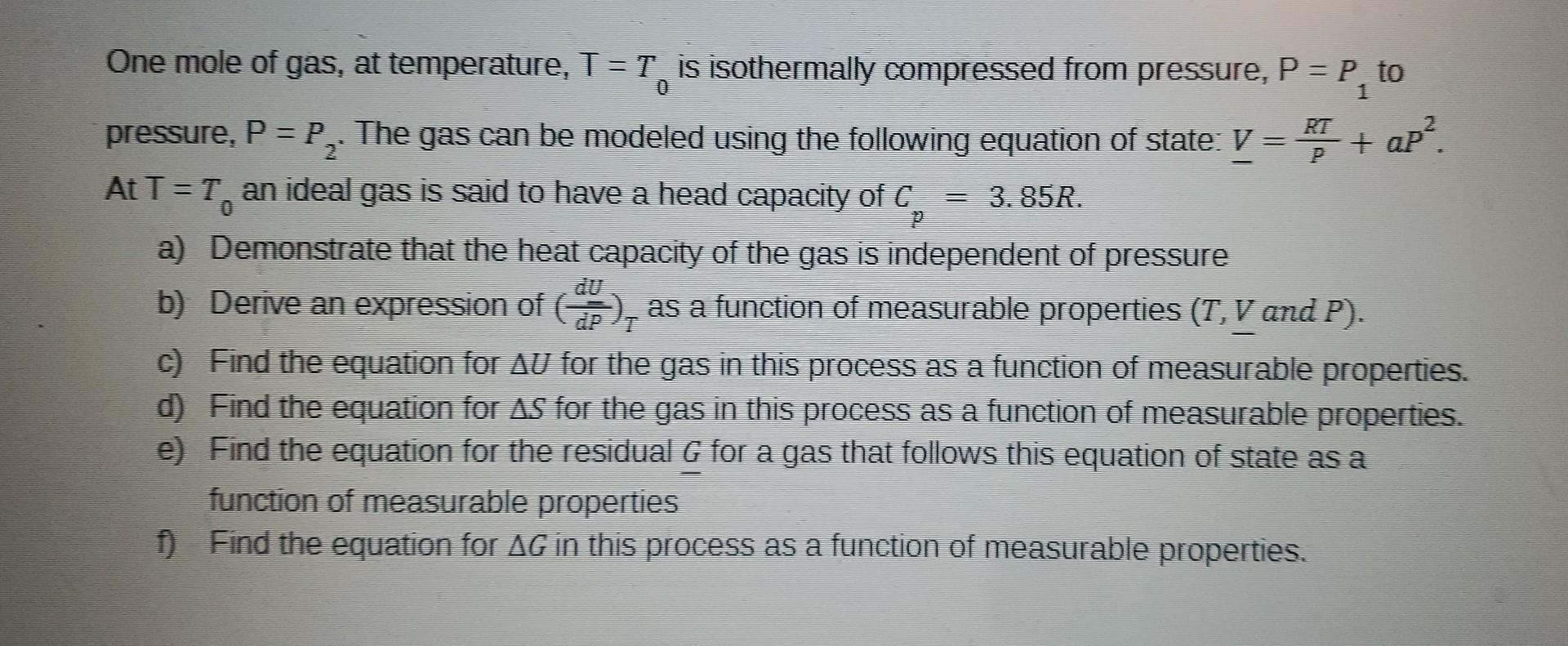
please answer the above question (all parts)
One mole of gas, at temperature, T=T0 is isothermally compressed from pressure, P=P1 to pressure, P=P2. The gas can be modeled using the following equation of state: V=PRT+aP2. At T=T0 an ideal gas is said to have a head capacity of Cp=3.85R. a) Demonstrate that the heat capacity of the gas is independent of pressure b) Derive an expression of (dPdU)T as a function of measurable properties (T,V and P). c) Find the equation for U for the gas in this process as a function of measurable properties. d) Find the equation for S for the gas in this process as a function of measurable properties. e) Find the equation for the residual G for a gas that follows this equation of state as a function of measurable properties f) Find the equation for G in this process as a function of measurable propertiesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


