Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted! Problem 1 (50 points) The following reactions
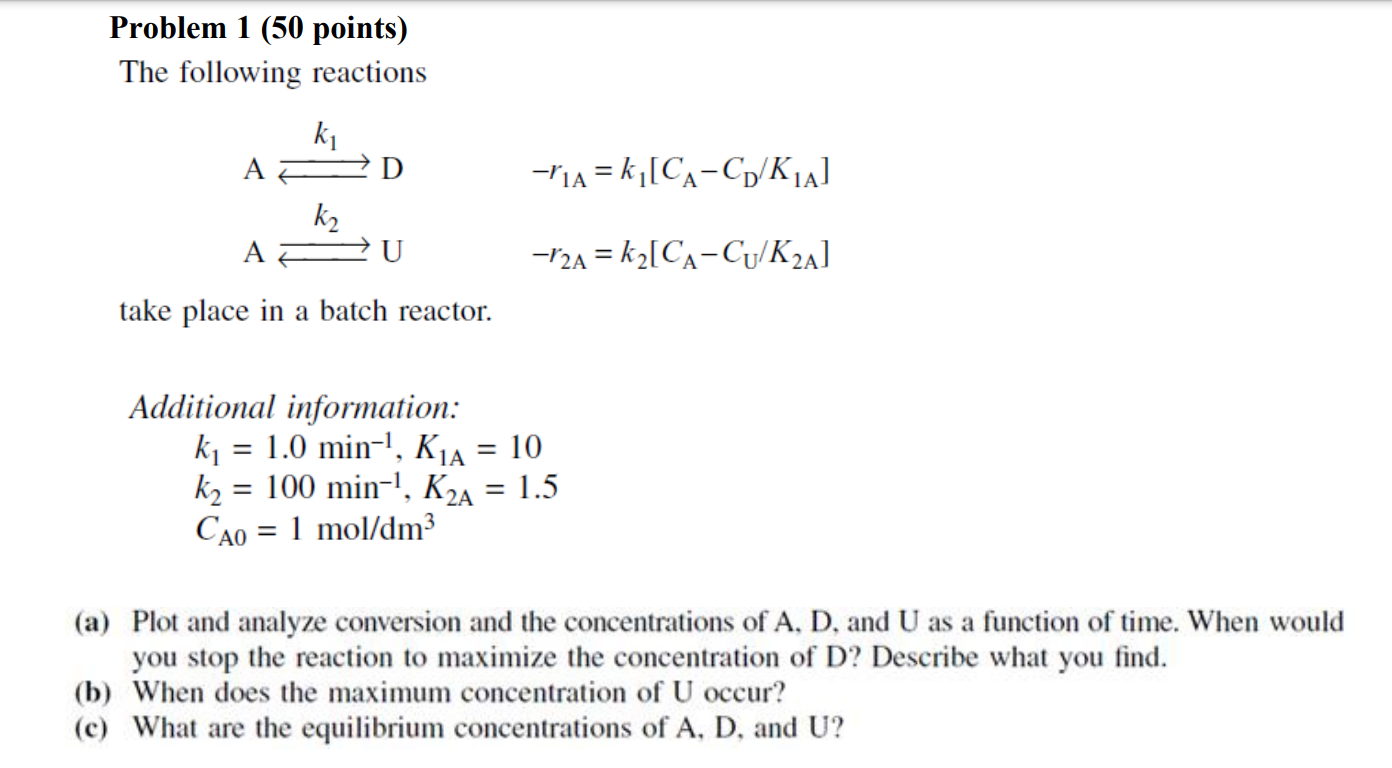
Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted!
Problem 1 (50 points) The following reactions Ak1LAk2Ur1A=k1[CACD/K1A]r2A=k2[CACU/K2A] take place in a batch reactor. Additional information: k1=1.0min1,K1A=10k2=100min1,K2A=1.5CA0=1mol/dm3 (a) Plot and analyze conversion and the concentrations of A, D, and U as a function of time. When would you stop the reaction to maximize the concentration of D ? Describe what you find. (b) When does the maximum concentration of U occur? (c) What are the equilibrium concentrations of A,D, and UStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


