Question
Please answer the following questions and refer to the figures below: 1. Analyze the intensity of the copper x-radiation as a function of the Bragg
Please answer the following questions and refer to the figures below:
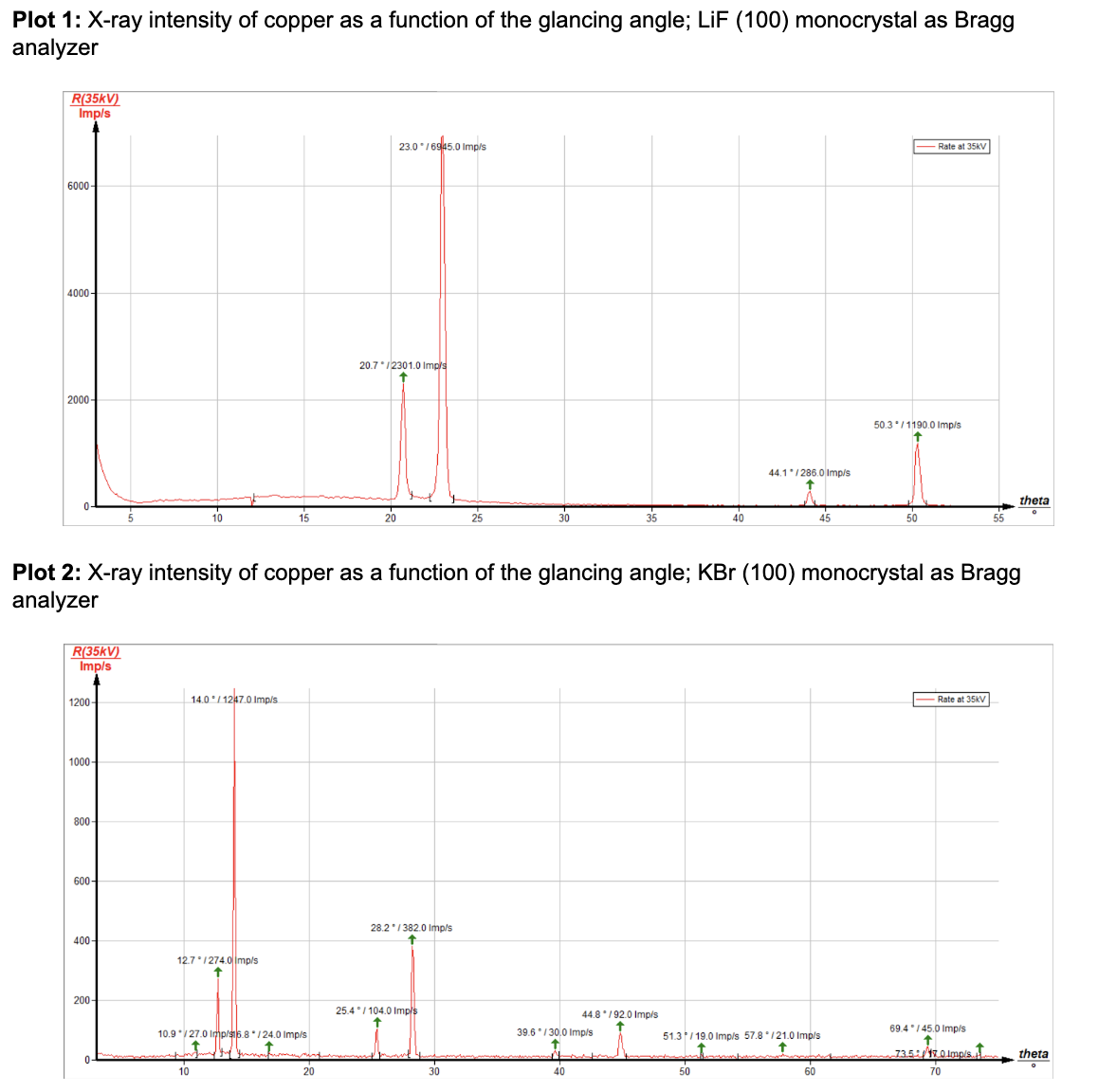
1. Analyze the intensity of the copper x-radiation as a function of the Bragg angle and with the aid of a LiF monocrystal
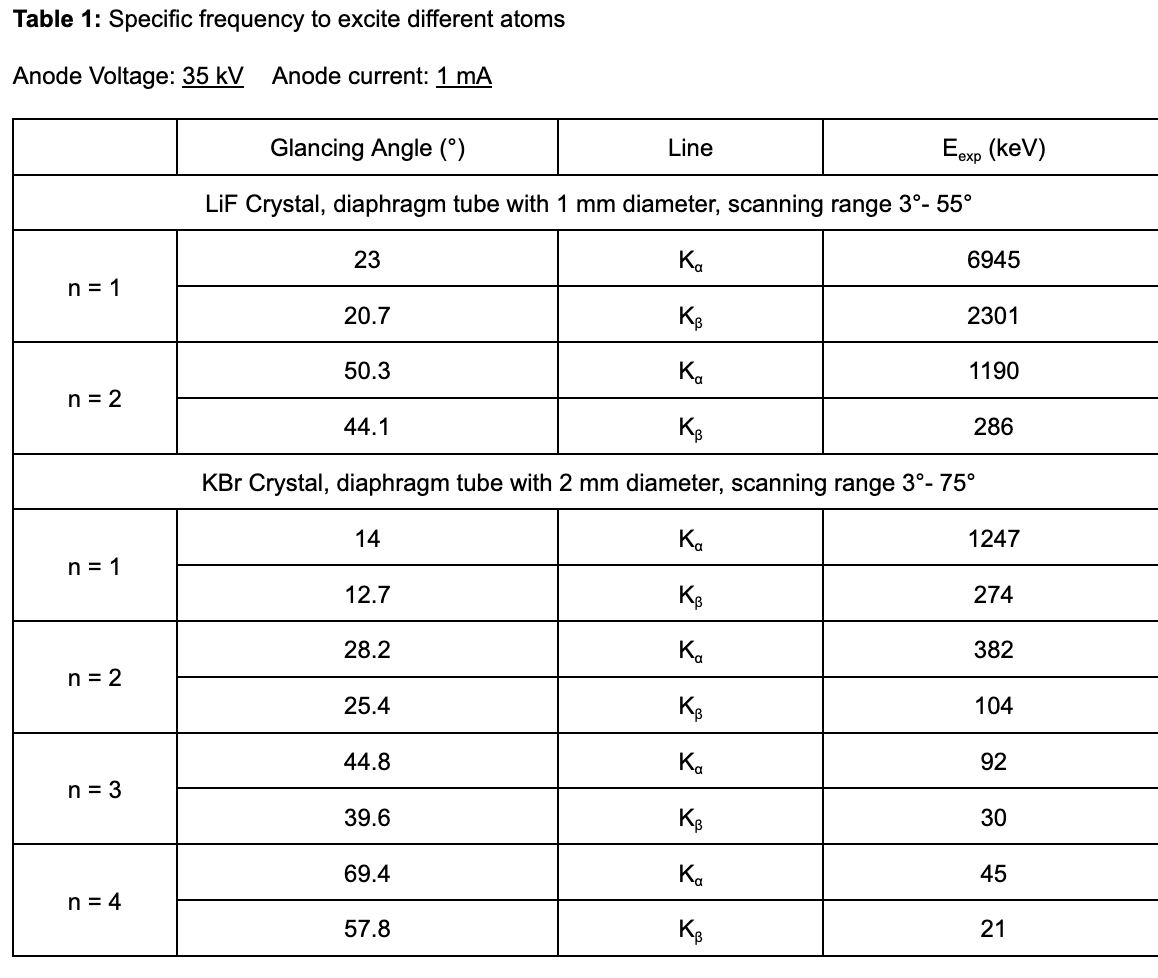
2. Analyze the intensity of copper x-radiation as a function of the Bragg angle with the aid of a KBr monocrystal
3. Determine the energy values of the characteristic x-rays of copper and compare them with the values that were determined based on the corresponding energy level diagram. Do so by completing the table below using the obtained spectra from the experiment
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


