Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer the question fully and write legibly 2. Membrane reactor. The reversible first order reaction ASB+ 2 C is carried out in the gas
Please answer the question fully and write legibly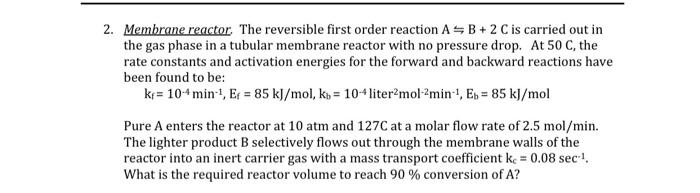
2. Membrane reactor. The reversible first order reaction ASB+ 2 C is carried out in the gas phase in a tubular membrane reactor with no pressure drop. At 50 C, the rate constants and activation energies for the forward and backward reactions have been found to be: kr = 104 min-1, Ep = 85 kJ/mol, ko = 10-4 liter molamin', E. = 85 kJ/mol Pure A enters the reactor at 10 atm and 127C at a molar flow rate of 2.5 mol/min. The lighter product B selectively flows out through the membrane walls of the reactor into an inert carrier gas with a mass transport coefficient k = 0.08 sec ! What is the required reactor volume to reach 90 % conversion of A 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


