Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer them, its urgent! The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea=6.30kJ/mol and a
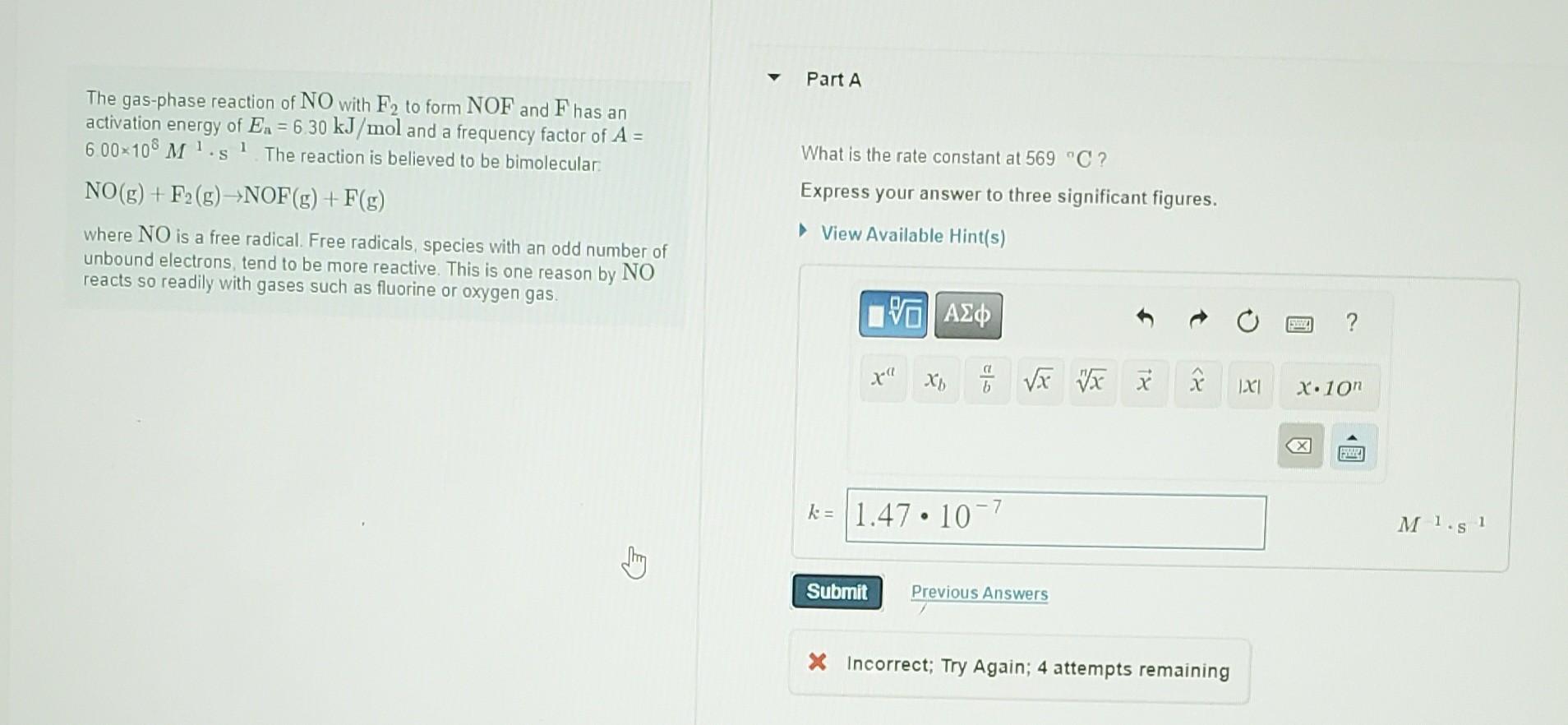
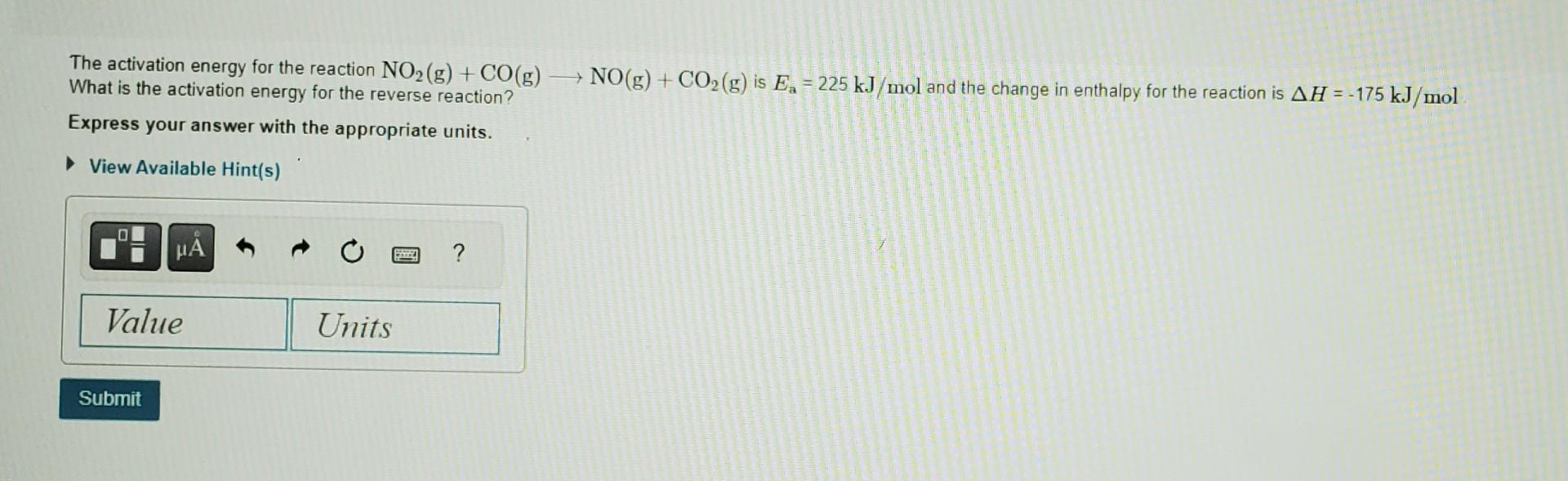
please answer them, its urgent!
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea=6.30kJ/mol and a frequency factor of A= 600108M1s1. The reaction is believed to be bimolecular: What is the rate constant at 569C ? NO(g)+F2(g)NOF(g)+F(g) Express your answer to three significant figures. where NO is a free radical. Free radicals, species with an odd number of unbound electrons, tend to be more reactive. This is one reason by NO reacts so readily with gases such as fluorine or oxygen gas. The activation energy for the reaction NO2(g)+CO(g)NO(g)+CO2(g) is Ea=225kJ/mol and the change in enthalpy for the reaction is H=175kJ/mol What is the activation energy for the reverse reaction? Express your answer with the appropriate unitsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


