Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE ANSWER THESE QUESTIONS.!!! You are provided with the following enthalpies: Hdissociation[Cl2]=121.5kJ/molHelectronaffinity[Cl(g)]=348.5kJ/molHionisationenergy[K(g)]=415.0kJ/molHatomisation[K(s)]=90.14kJ/molHcystallisation[KCl(s)]=708kJ/mol 25. Fill in the blanks in the Born Haber cycle below to show
PLEASE ANSWER THESE QUESTIONS.!!! 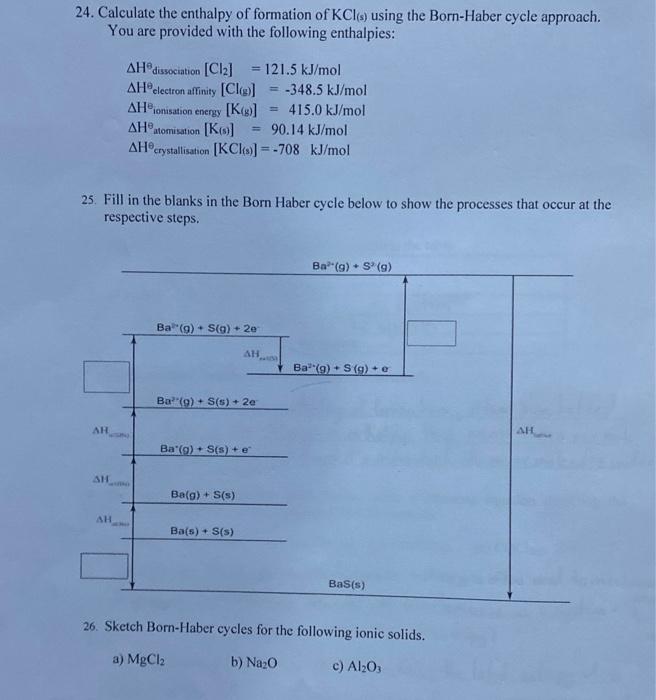
You are provided with the following enthalpies: Hdissociation[Cl2]=121.5kJ/molHelectronaffinity[Cl(g)]=348.5kJ/molHionisationenergy[K(g)]=415.0kJ/molHatomisation[K(s)]=90.14kJ/molHcystallisation[KCl(s)]=708kJ/mol 25. Fill in the blanks in the Born Haber cycle below to show the processes that occur at the respective steps. 26. Sketch Born-Haber cycles for the following ionic solids. a) MgCl2 b) Na2O c) Al2O3 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


