Answered step by step
Verified Expert Solution
Question
1 Approved Answer
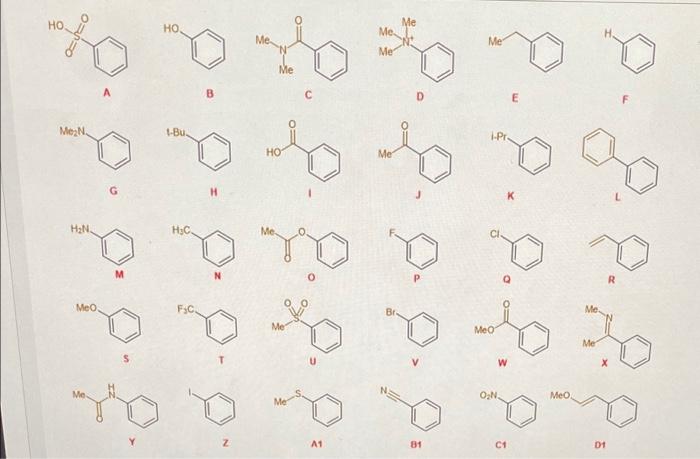
Please answer this multiple part question. The answers to choose from are as follows: 1. EDG 2. EWG 3. electron-neutral Inspect the benzene rings below.
Please answer this multiple part question. The answers to choose from are as follows:
Inspect the benzene rings below. Each has a substituent on it in tan/brown. Please indicate whether you think the substituents would act as electron-donating groups (EDGs), electron-withdrawing groups (EWGs), or electron-neutral groups. Here is how I would like you to think about this. Start by asking yourselves the following questions: 1). Can the indicated group interact with the benzene ring through resonance? If so, does it donate or withdraw electrons? Depending on your answer here, you can clearly assign it as one of the three possibilities. 2). If the indicated group CANNOT interact with the benzene ring through resonance, then ask yourself if it is electronwithdrawing through inductive effects (in which case it would be an EWG)? Or, can it donate electrons through hyperconjugation (in which case it would be an EDG)? 3). If neither resonance nor inductive/hyperconjugation effects allows you to identify it as donating or withdrawing, then it is likely an electron neutral group. Alternatively, if a group can BOTH donate and accept electrons through the effects discussed above, then it is likely also an electron neutral group (as the effects would likely cancel each other out). 4). If resonance effects suggest one thing, but inductivehyperconjugation effects suggest a different thing, the former (resonance) will always trump 1. EDG
2. EWG
3. electron-neutral 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


