Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the elementary reaction: 2A B 1.1 If the reaction is elementary and irreversible, what is the rate of reaction? (2] 1.2 What is
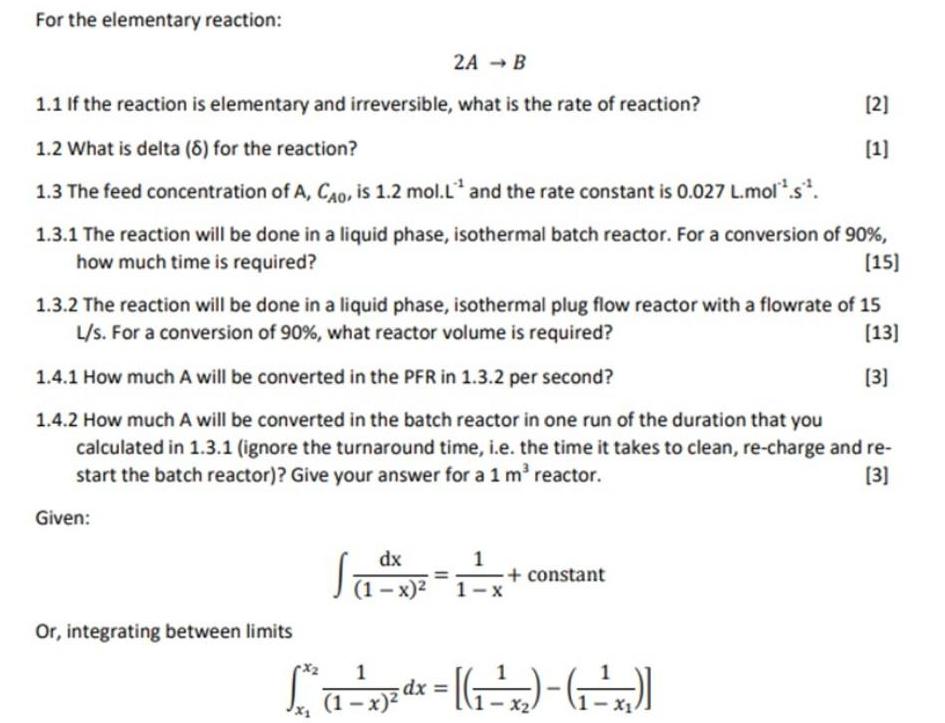
For the elementary reaction: 2A B 1.1 If the reaction is elementary and irreversible, what is the rate of reaction? (2] 1.2 What is delta (8) for the reaction? (1] 1.3 The feed concentration of A, CAO, is 1.2 mol.L and the rate constant is 0.027 L.mol*.s*. 1.3.1 The reaction will be done in a liquid phase, isothermal batch reactor. For a conversion of 90 %, [15] how much time is required? 1.3.2 The reaction will be done in a liquid phase, isothermal plug flow reactor with a flowrate of 15 L/s. For a conversion of 90%, what reactor volume is required? (13] 1.4.1 How much A will be converted in the PFR in 1.3.2 per second? [3] 1.4.2 How much A will be converted in the batch reactor in one run of the duration that you calculated in 1.3.1 (ignore the turnaround time, i.e. the time it takes to clean, re-charge and re- start the batch reactor)? Give your answer for a 1 m' reactor. (3] Given: dx | 1- x) 1 + constant Or, integrating between limits dx = (1-x)2 - X2 1- x1
Step by Step Solution
★★★★★
3.31 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


