Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemical separation system is used to separate benzene, styrene, toluene, and xylene with an array of distillation columns shown in Figure 1. Experimental
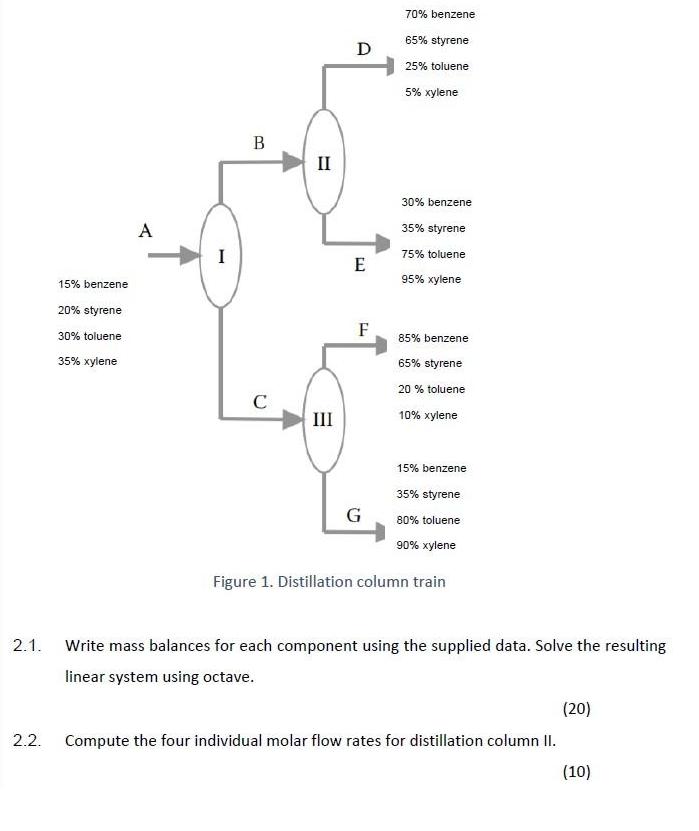
A chemical separation system is used to separate benzene, styrene, toluene, and xylene with an array of distillation columns shown in Figure 1. Experimental data show that the feed rate (stream A) is 100 mole/hr and that the composition of stream A is 15% benzene, 20% styrene, 30% toluene and 35% xylene (compositions are in mole %). The following information is available for this system: i. 70% benzene, 65% styrene, 25% toluene and 5% xylene of stream B go overhead to stream D. The remainder goes out the bottom to stream E. ii. 85% benzene, 60% styrene, 20% toluene and 10% xylene of stream C go overhead to stream F. The remainder goes out the bottom to stream G. Use OCTAVE or MATLAB coding. 70% benzene 65% styrene D 25% toluene 5% xylene II 30% benzene A 35% styrene I 75% toluene E 15% benzene 95% xylene 20% styrene F 30% toluene 85% benzene 35% xylene 65% styrene 20 % toluene C III 10% xylene 15% benzene 35% styrene G 80% toluene 90% xylene Figure 1. Distillation column train 2.1. Write mass balances for each component using the supplied data. Solve the resulting linear system using octave. (20) 2.2. Compute the four individual molar flow rates for distillation column II. (10)
Step by Step Solution
★★★★★
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Compute the four individual molar flow rates for distillation column II Molar Fl...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6360abd1da222_229358.pdf
180 KBs PDF File
6360abd1da222_229358.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


