Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answere soon The chemical reaction that causes magnesium to The standard Gbbs energy change, tG, applies only when the reactants and products are in
please answere soon 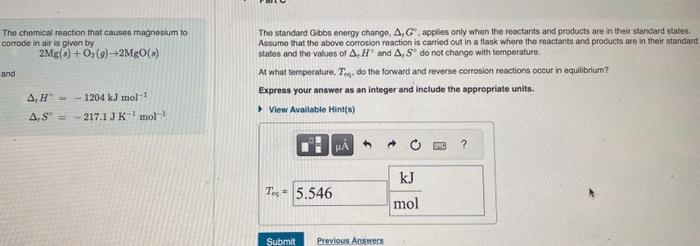
The chemical reaction that causes magnesium to The standard Gbbs energy change, tG, applies only when the reactants and products are in their standard states. corrode in air is given by Assume that the above corrosion reaction is carried out in a flasik whore the reactants and products are in their standare 2Mg(s)+O2(g)2MgO(s) states and the values of TH and rS do not change with temperature. At what tempecature, Teq, do the forward and reverse corresion reactions occur in equilibrium? rH2=1204kJmol1rS=217.1JK1mol1 Express your answer as an integer and include the apprepriate units 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


