Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answers for 3 problems. 1. Gallium consists of two isotopes of masses 68.95 amu and 70.95 amu with abundance of 60.16% and 39.84%, respectively.
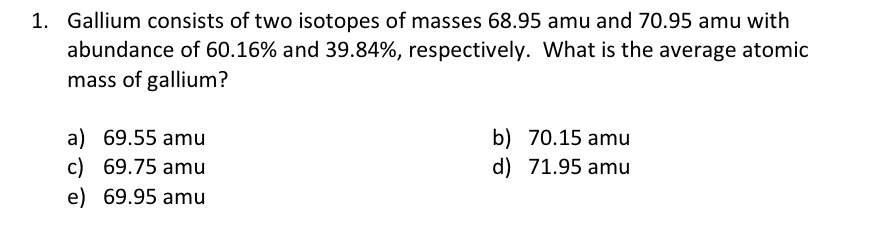


Please answers for 3 problems.
1. Gallium consists of two isotopes of masses 68.95 amu and 70.95 amu with abundance of 60.16% and 39.84%, respectively. What is the average atomic mass of gallium? a) 69.55amu b) 70.15amu c) 69.75amu d) 71.95amu e) 69.95amu 5. Which of the following statement describes Molecular compound the best? a) A compound that is made up of a metal and a non-metal atoms. b) A compound that is made up of a metal and a non-metal atoms with a covalent bond between them. c) A compound that is made up of two or more non-metal atoms with an ionic bond between atoms. d) A compound that is made up of two or more non-metal atoms with a covalent bond between atoms. 6. State what types of bonds exist between NO,HH, and HF. a) H-bonding, True Covalent bond and Polar covalent bond b) Ionic bond, True Covalent bond and Polar covalent bond c) Polar covalent bond, True covalent bond, and Ionic bond d) Ionic bond, true covalent bond, and true covalent bondStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


