Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answers with some explaination Figure Q1 below shows the cycle followed by one mole of a perfect gas. Assuming each process is carried out
please answers with some explaination 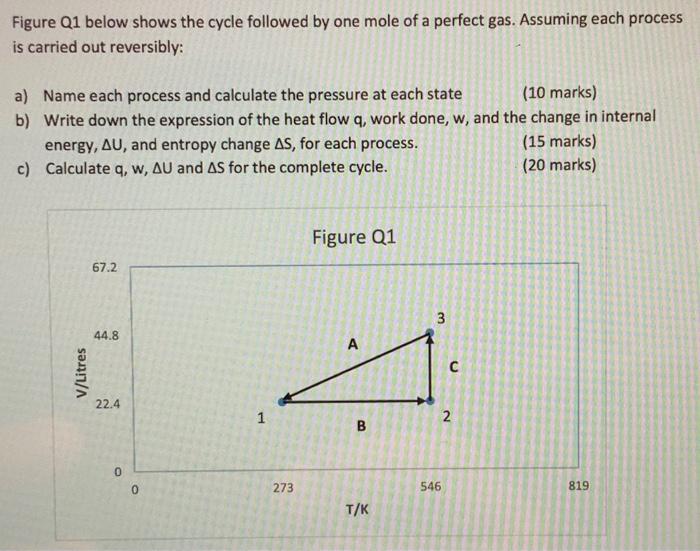
Figure Q1 below shows the cycle followed by one mole of a perfect gas. Assuming each process is carried out reversibly: a) Name each process and calculate the pressure at each state (10 marks) b) Write down the expression of the heat flow q, work done, w, and the change in internal energy, AU, and entropy change AS, for each process. (15 marks) c) Calculate q, w, AU and As for the complete cycle. (20 marks) Figure Q1 67.2 3 3 44.8 A V/Litres 22.4 1 N B 0 0 273 546 819 T/K 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


