Answered step by step
Verified Expert Solution
Question
1 Approved Answer
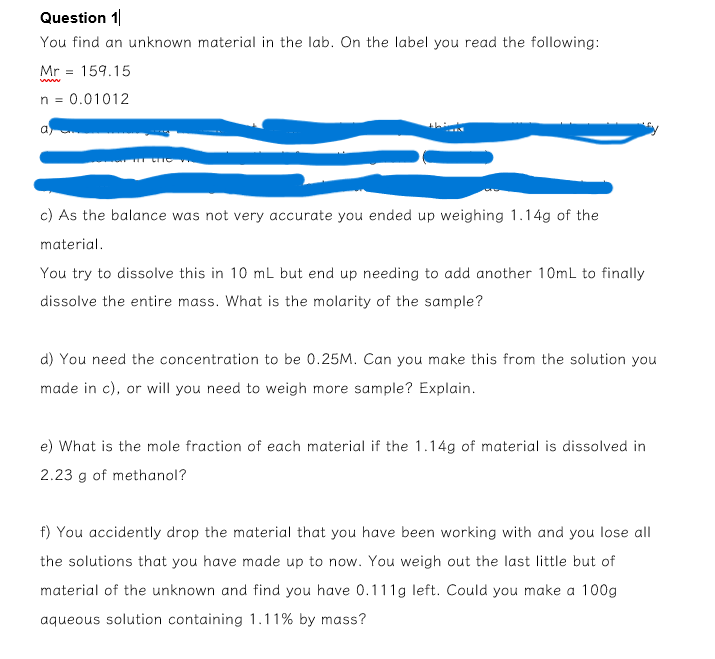
PLEASE ASSIST URGENTLY :Question 1 | You find an unknown material in the lab. On the label you read the following: M r = 1
PLEASE ASSIST URGENTLY :Question
You find an unknown material in the lab. On the label you read the following:
c As the balance was not very accurate you ended up weighing of the
material.
You try to dissolve this in but end up needing to add another to finally
dissolve the entire mass. What is the molarity of the sample?
d You need the concentration to be Can you make this from the solution you
made in c or will you need to weigh more sample? Explain.
e What is the mole fraction of each material if the of material is dissolved in
of methanol?
f You accidently drop the material that you have been working with and you lose all
the solutions that you have made up to now. You weigh out the last little but of
material of the unknown and find you have left. Could you make a
aqueous solution containing by mass?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


