Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please assist with determining the conversion of propylene in both reactions listed above. Use 300 degrees celcius as the basis calc for both reactions. Need
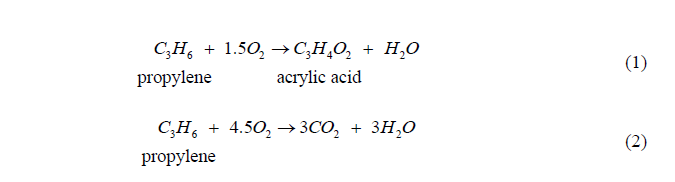
Please assist with determining the conversion of propylene in both reactions listed above.
Use 300 degrees celcius as the basis calc for both reactions.
Need to have conversion % for both reactions to run a simulation.
First reaction is the desired product of Acrylic acid forming
Second reaction is the side reaction where CO2 is formed.
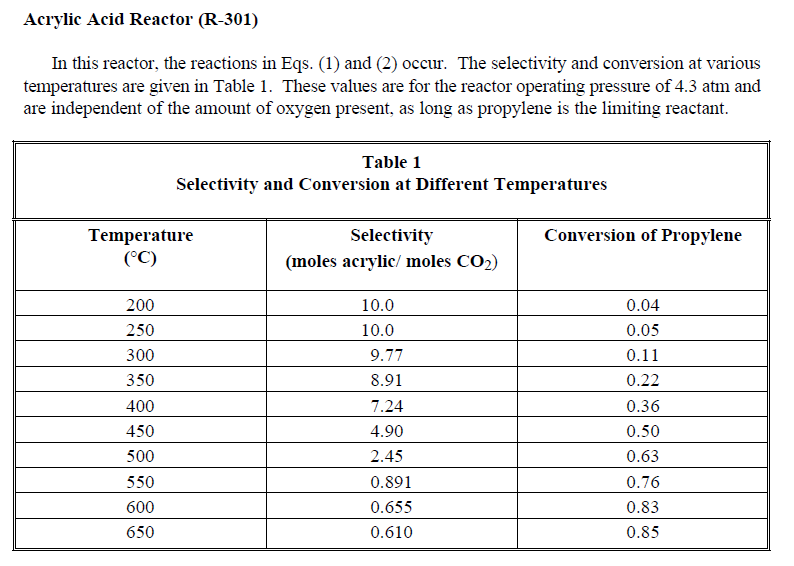
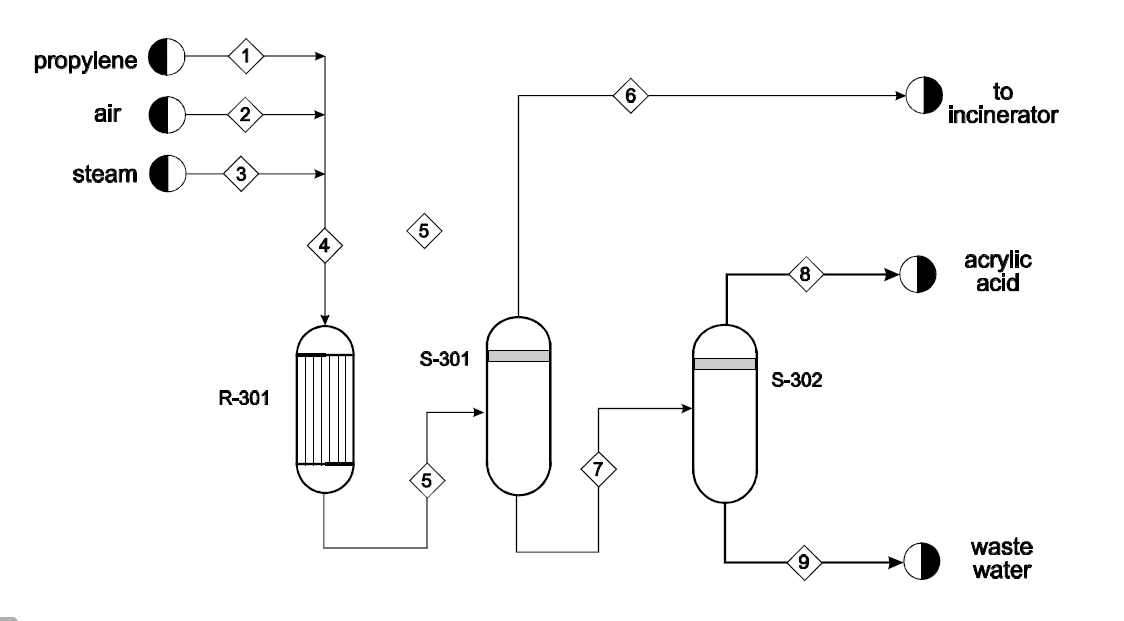
C3H6+1.5O2C3H4O2+H2O propylene acrylic acid C3H6+4.5O23CO2+3H2O Acrylic Acid Reactor (R-301) In this reactor, the reactions in Eqs. (1) and (2) occur. The selectivity and conversion at various temperatures are given in Table 1. These values are for the reactor operating pressure of 4.3atm and are independent of the amount of oxygen present, as long as propylene is the limiting reactant. propylene to air incinerator steam acrylic acid waste water
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


