Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please check work! 2. (40 pts) Now you need tag these waste buckets summarizing the contents. Fill out the two tags shown below and show
Please check work!
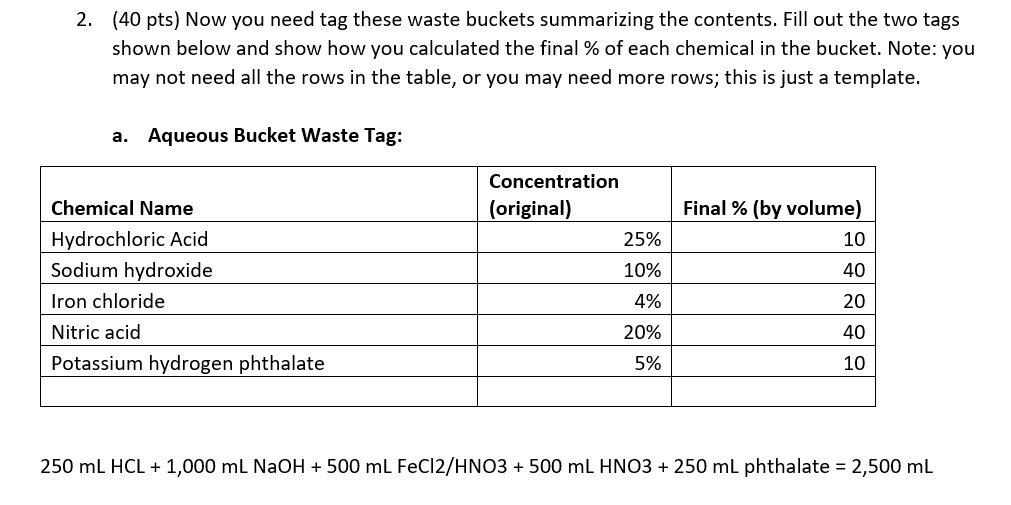
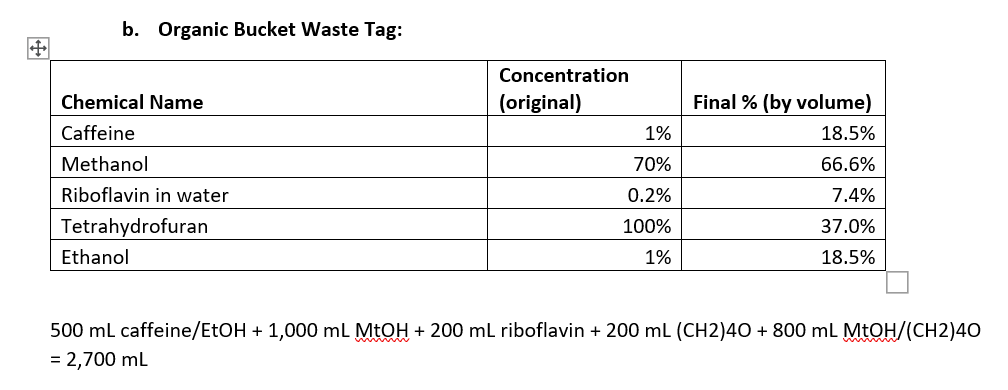
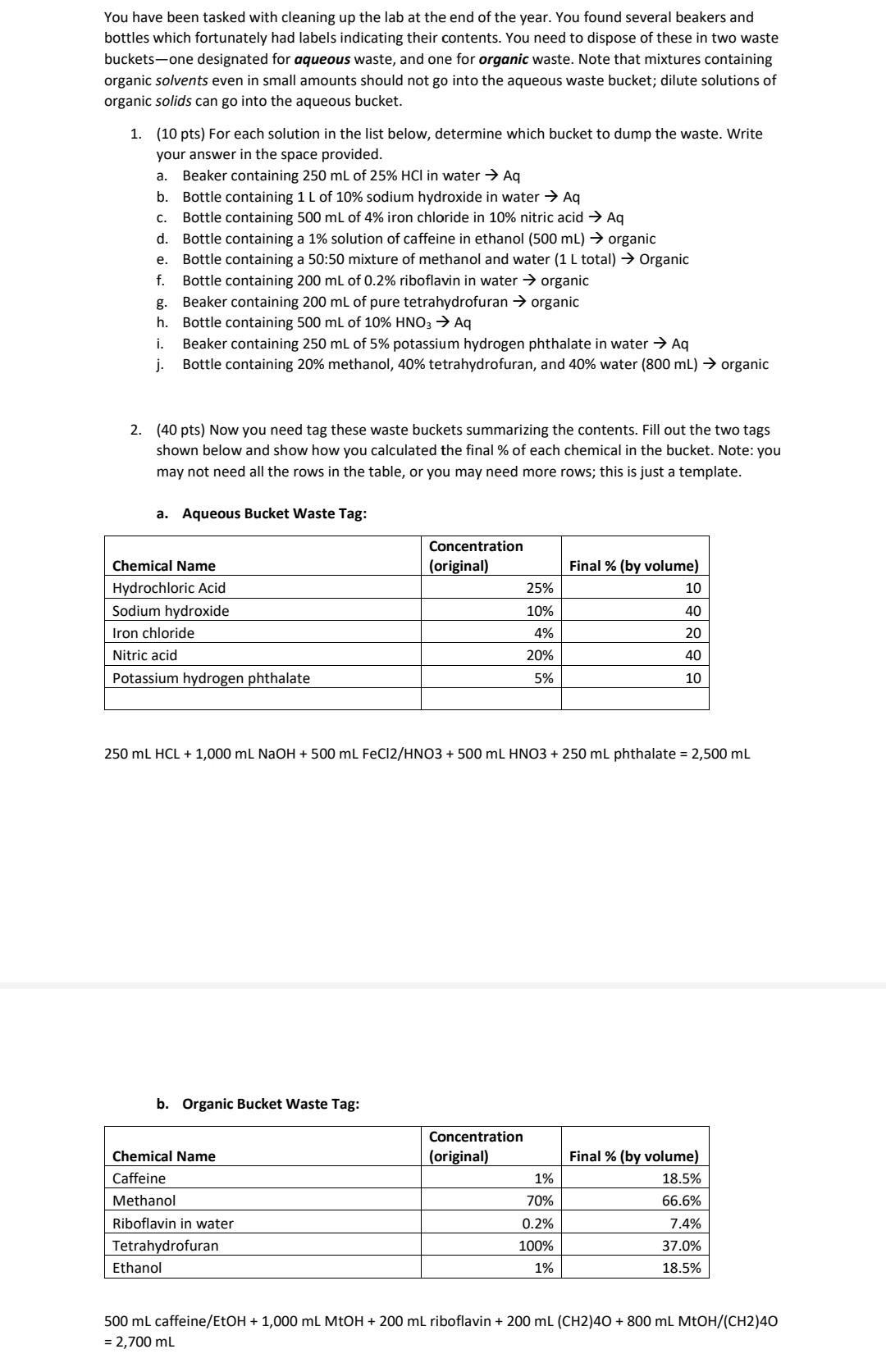
2. (40 pts) Now you need tag these waste buckets summarizing the contents. Fill out the two tags shown below and show how you calculated the final \% of each chemical in the bucket. Note: you may not need all the rows in the table, or you may need more rows; this is just a template. a. Aqueous Bucket Waste Tag: 250mLHCL+1,000mLNaOH+500mLFeCl2/HNO3+500mLHNO3+250mL phthalate =2,500mL b. Organic Bucket Waste Tag: 500mLcaffeine/EtOH+1,000mLMtOH+200mLriboflavin+200mL(CH2)4O+800mLMtOH/(CH2)40=2,700mL You have been tasked with cleaning up the lab at the end of the year. You found several beakers and bottles which fortunately had labels indicating their contents. You need to dispose of these in two waste buckets-one designated for aqueous waste, and one for organic waste. Note that mixtures containing organic solvents even in small amounts should not go into the aqueous waste bucket; dilute solutions of organic solids can go into the aqueous bucket. 1. (10 pts) For each solution in the list below, determine which bucket to dump the waste. Write your answer in the space provided. a. Beaker containing 250mL of 25%HCl in water Aq b. Bottle containing 1L of 10% sodium hydroxide in water Aq c. Bottle containing 500mL of 4% iron chloride in 10% nitric acid Aq d. Bottle containing a 1% solution of caffeine in ethanol (500mL) organic e. Bottle containing a 50:50 mixture of methanol and water (1L total) Organic f. Bottle containing 200mL of 0.2% riboflavin in water organic g. Beaker containing 200mL of pure tetrahydrofuran organic h. Bottle containing 500mL of 10%HNO3Aq i. Beaker containing 250mL of 5% potassium hydrogen phthalate in water Aq j. Bottle containing 20% methanol, 40% tetrahydrofuran, and 40% water (800mL) organic 2. (40 pts) Now you need tag these waste buckets summarizing the contents. Fill out the two tags shown below and show how you calculated the final \% of each chemical in the bucket. Note: you may not need all the rows in the table, or you may need more rows; this is just a template. a. Aqueous Bucket Waste Tag: 250mLHCL+1,000mLNaOH+500mLFeCl2/HNO+500mLHNO3+250mL phthalate =2,500mL b. Organic Bucket Waste Tag: 500mLcaffeine/EtOH+1,000mLMtOH+200mLriboflavin+200mL(CH2)4O+800mLMtOH/(CH2)40=2,700mL
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


