Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please complete table 5.1, 5.2 , 5.3 , 5.4, 5.5 and all the questions ask in the sheets. ps: complete all the tables and all
please complete table 5.1, 5.2 , 5.3 , 5.4, 5.5 and all the questions ask in the sheets. 



ps: complete all the tables and all the questions asked . please make it legible and net. Thank you.. all the informations are posted nothing is missing.
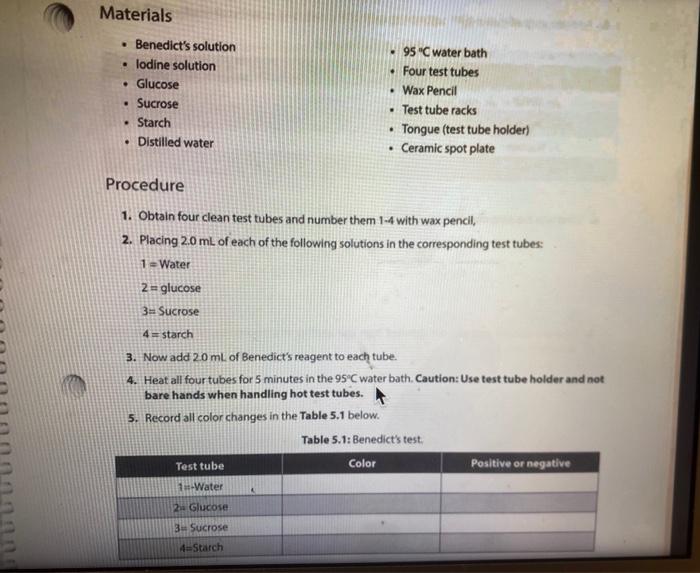
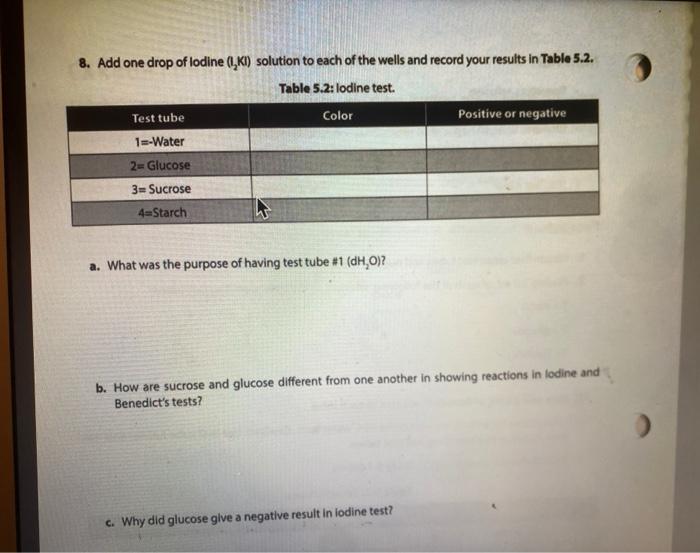
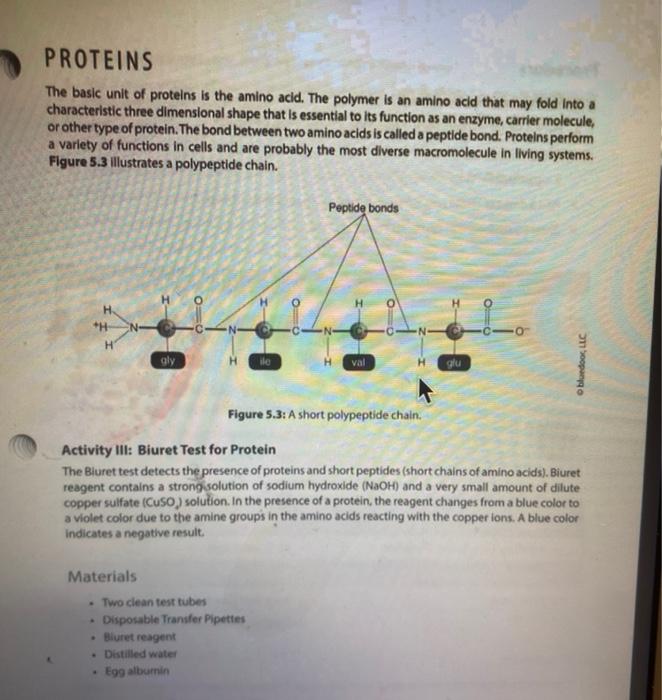
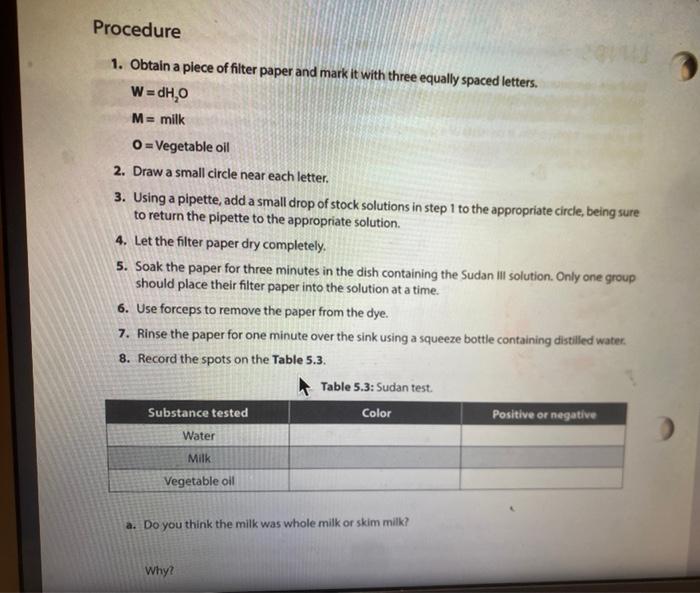
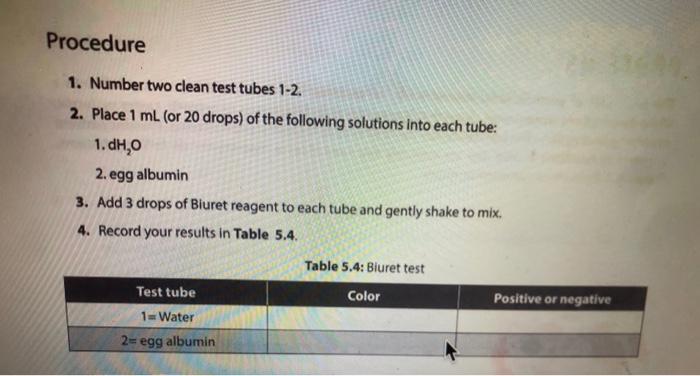
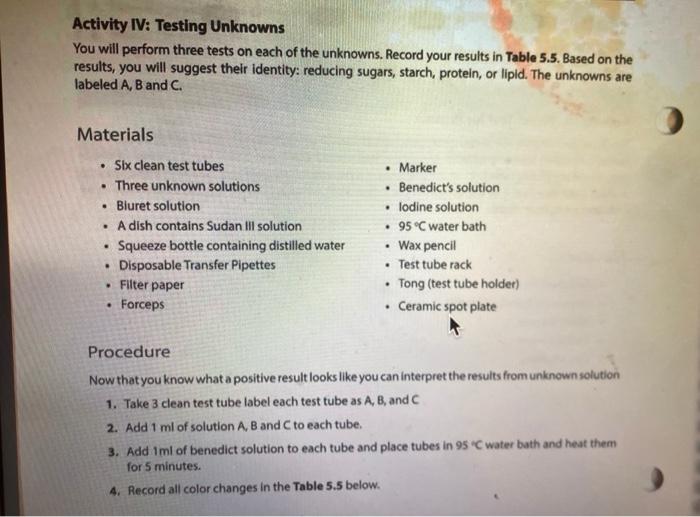
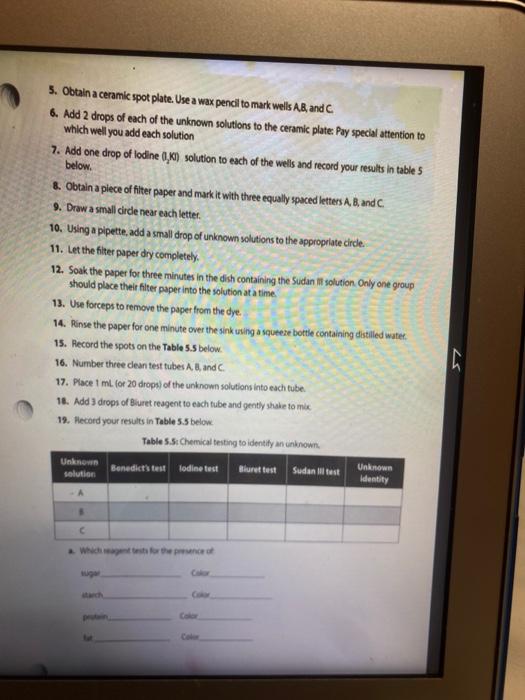
Activity l: Benedict's and lodine Tests The Benedict's test detects the presence of reducing sugars. Reducing sugars are usually single sugars or disaccharides, which have a free aldehyde group (-CHO) that can be oxidized. Generally, large polysaccharides are non-reducing sugars. When heated to boiling in the presence of Benedict's reagent, reducing sugars will turn the reagent green (indicating low concentration), or yellow, orange, or red-orange (indicating high concentrations), depending on the concentration of reducing sugars present. If no reducing sugar is present, the Benedict's reagent stays blue. The iodine (TK) test is a carbohydrate test that identifies the presence of starch. Starch molecules are the polymers of glucose commonly found in plants. In the presence of starch, the color of iodine changes from Yellow-Brown to bue-black. If the test is negative, the solution stays Yellow Brown. Materials Benedict's solution lodine solution Glucose Sucrose Starch Distilled water 95 " water bath Four test tubes Wax Pencil Test tube racks Tongue (test tube holder) Ceramic spot plate . . Procedure 1. Obtain four clean test tubes and number them 1-4 with wax pencil, 2. Placing 2.0 mL of each of the following solutions in the corresponding test tubes: 1 = Water 2 = glucose 3= Sucrose 4= starch 3. Now add 20 ml. of Benedict's reagent to each tube. 4. Heat all four tubes for 5 minutes in the 95C water bath Caution: Use test tube holder and not bare hands when handling hot test tubes. 5. Record all color changes in the Table 5.1 below. Table 5.1: Benedict's test. Test tube Color Positive or negative 1 Water 2 Glucose 3. Sucrose 4-Starch 6. Obtain a ceramic spot plate. Use a wax pencil to mark wells 1-4. 7. Add 2 drops of each of the following solutions to the ceramic plate: 1. water 2. glucose 3. Sucrose 4. starch (Make sure you record of which well you add each solution.) 8. Add one drop of lodine (1,KI) solution to each of the wells and record your results in Table 5.2. Table 5.2: lodine test. Test tube Color Positive or negative 1=-Water 2-Glucose 3= Sucrose 4-Starch a. What was the purpose of having test tube #1 (dH,0)? b. How are sucrose and glucose different from one another in showing reactions in lodine and Benedict's tests? c. Why did glucose give a negative result in lodine test? PROTEINS The basic unit of proteins is the amino acid. The polymer is an amino acid that may fold Into characteristic three dimensional shape that is essential to its function as an enzyme, carrier molecule, or other type of protein. The bond between two amino acids is called a peptide bond. Proteins perform a variety of functions in cells and are probably the most diverse macromolecule in living systems. Figure 5.3 illustrates a polypeptide chain. Peptide bonds H *H H 0 gly val glu boopanga Figure 5.3: A short polypeptide chain. Activity Ill: Biuret Test for Protein The Bluret test detects the presence of proteins and short peptides (short chains of amino acids). Biuret reagent contains a strong solution of sodium hydroxide (NaOH) and a very small amount of dilute copper sulfate (Cuso) solution. In the presence of a protein, the reagent changes from a blue color to a violet color due to the amine groups in the amino acids reacting with the copper ions. A blue color indicates a negative result Materials . Two clean test tubes Disposable Transfer Pipettes Bluret reagent Distilled water Egg albumin Procedure 1. Obtain a piece of filter paper and mark it with three equally spaced letters. W = dH,0 M = milk 0 = Vegetable oil 2. Draw a small circle near each letter. 3. Using a pipette, add a small drop of stock solutions in step 1 to the appropriate circle, being sure to return the pipette to the appropriate solution 4. Let the filter paper dry completely. 5. Soak the paper for three minutes in the dish containing the Sudan Ill solution. Only one group should place their filter paper into the solution at a time. 6. Use forceps to remove the paper from the dye. 7. Rinse the paper for one minute over the sink using a squeeze bottle containing distilled water. 8. Record the spots on the Table 5.3. Table 5.3: Sudan test Positive or negative Substance tested Color Water Milk Vegetable oil a. Do you think the milk was whole milk or skim milk? Why? PROTEINS The basic unit of proteins is the amino acid. The polymer is an amino acid that may fold into a characteristic three dimensional shape that is essential to its function as an enzyme carrier molecule, or other type of protein. The bond between two amino acids is called a peptide bond. Proteins perform a variety of functions in cells and are probably the most diverse macromolecule in living systems. Figure 5.3 illustrates a polypeptide chain. Peptide bonds "H H gly do val bluedoor lic glu Figure 5.3: A short polypeptide chain. Activity Ill: Biuret Test for Protein The Biuret test detects the presence of proteins and short peptides (short chains of amino acids). Biuret reagent contains a strong solution of sodium hydroxide (NaOH) and a very small amount of dilute copper sulfate (Cuso) solution. In the presence of a protein, the reagent changes from a blue color to a violet color due to the amine groups in the amino acids reacting with the copper ions. A blue color indicates a negative result. Materials Two clean test tubes Disposable Transfer Pipettes .Bluret reagent Distiled water Procedure 1. Number two clean test tubes 1-2. 2. Place 1 mL (or 20 drops) of the following solutions into each tube: 1. d4,0 2. egg albumin 3. Add 3 drops of Biuret reagent to each tube and gently shake to mix. 4. Record your results in Table 5.4. Table 5.4: Biuret test Test tube Color Positive or negative 1 Water 2= egg albumin Activity IV: Testing Unknowns You will perform three tests on each of the unknowns. Record your results in Table 5.5. Based on the results, you will suggest their identity: reducing sugars, starch, protein, or lipid. The unknowns are labeled A, B and C. Materials . Six clean test tubes Three unknown solutions Bluret solution . A dish contains Sudan Ill solution Squeeze bottle containing distilled water Disposable Transfer Pipettes Filter paper Forceps Marker Benedict's solution lodine solution . 95C water bath Wax pencil Test tube rack Tong (test tube holder) Ceramic spot plate . . . Procedure Now that you know what a positive result looks like you can interpret the results from unknown solution 1. Take 3 clean test tube label each test tube as A, B, and C 2. Add 1 ml of solution A, B and C to each tube. 3. Add Iml of benedict solution to each tube and place tubes in 95 C water bath and heat them for 5 minutes. 4. Record all color changes in the Table 5.5 below. 3. Obtain a ceramic spot plate. Use a wax pencil to mark wells AB, and 6. Add 2 drops of each of the unknown solutions to the ceramic plate: Pay special attention to which well you add each solution 7. Add one drop of iodine (K) solution to each of the wells and record your results in tables below. & Obtain a piece of filter paper and mark it with three equally spaced letters A, B, and C. 9. Draw a small cirde near each letter. 10. Using a pipette, add a small drop of unknown solutions to the appropriate circle 11. Let the filter paper dry completely 12. Soak the paper for three minutes in the dish containing the Sudan 1 solution Only one group should place their filter paper into the solution at a time. 13. Use forceps to remove the paper from the dye. 14. Rinse the paper for one minute over the sink using a squeeze bottle containing distilled water. 15. Record the spots on the Table 5.5 below. 16. Number three dean test tubes AB and 17. Place 1 ml for 20 drops) of the unknown solutions into each tube 18. Add 3 drops of Biuret reagent to each tube and gently shake to me 19. Hecord your results in Table 5.5 below Table 5.5 Chemical testing to identity an unknown st Unknown solution Benedict's test lodine test Burettest Sudan Il test Unknown Identity 3 w Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


