Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please compute in excel. Or show equations needed for excel. Normal butane is to be isomerised to isobutane in a PFR. For this example, the
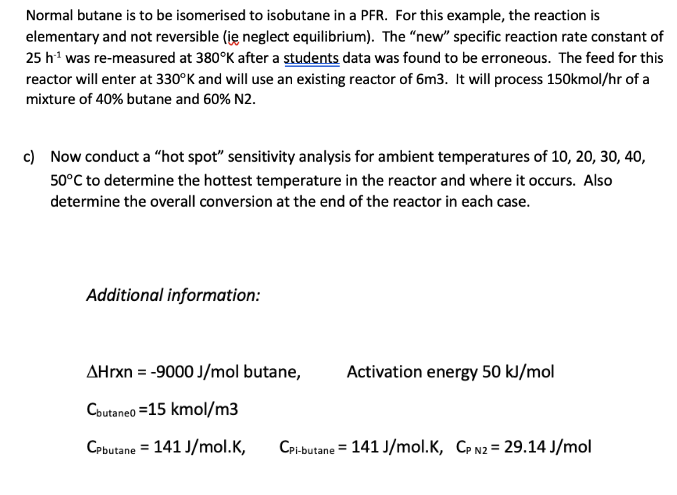
Please compute in excel. Or show equations needed for excel.
Normal butane is to be isomerised to isobutane in a PFR. For this example, the reaction is elementary and not reversible (ie neglect equilibrium). The "new" specific reaction rate constant of 25h1 was re-measured at 380K after a students data was found to be erroneous. The feed for this reactor will enter at 330K and will use an existing reactor of 6m3. It will process 150kmol/hr of a mixture of 40% butane and 60% N2. c) Now conduct a "hot spot" sensitivity analysis for ambient temperatures of 10, 20, 30, 40, 50C to determine the hottest temperature in the reactor and where it occurs. Also determine the overall conversion at the end of the reactor in each case. Additional information: Hrxn=9000J/mol butane, Activation energy 50kJ/mol Cbutaneo=15kmol/m3 CPbutane=141J/mol.K,CPi-butane=141J/mol.K,CPN2=29.14J/molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


