Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please could someone explain with answers. seem to be confused in where to start. thank you! EDTA forms coloured complexes with a variety of metal
please could someone explain with answers. seem to be confused in where to start. thank you! 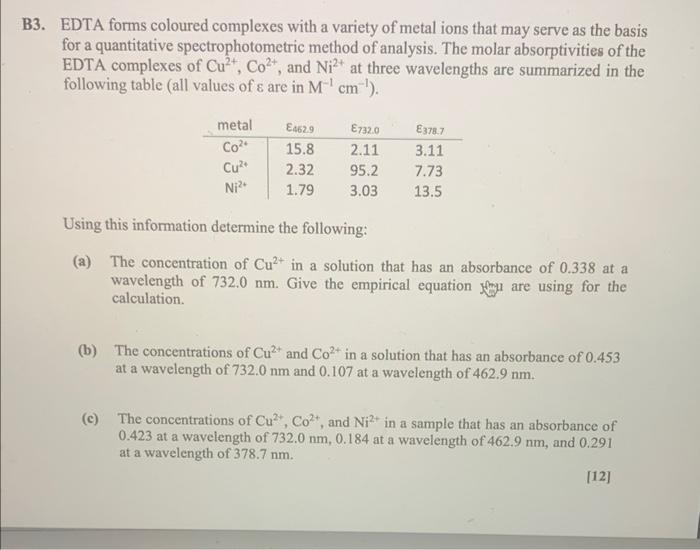
EDTA forms coloured complexes with a variety of metal ions that may serve as the basis for a quantitative spectrophotometric method of analysis. The molar absorptivities of the EDTA complexes of Cu2+,Co2+, and Ni2+ at three wavelengths are summarized in the following table (all values of are in M1cm1 ). Using this information determine the following: (a) The concentration of Cu2+ in a solution that has an absorbance of 0.338 at a wavelength of 732.0nm. Give the empirical equation fnn are using for the calculation. (b) The concentrations of Cu2+ and Co2+ in a solution that has an absorbance of 0.453 at a wavelength of 732.0nm and 0.107 at a wavelength of 462.9nm. (c) The concentrations of Cu2+,Co2+, and Ni2+ in a sample that has an absorbance of 0.423 at a wavelength of 732.0nm,0.184 at a wavelength of 462.9nm, and 0.291 at a wavelength of 378.7nm. [12] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


