Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please do it correctly.. 1. The irreversible isomerization reaction, A + B, was carried out in a batch reactor at 250C and 1 atm. The
please do it correctly..
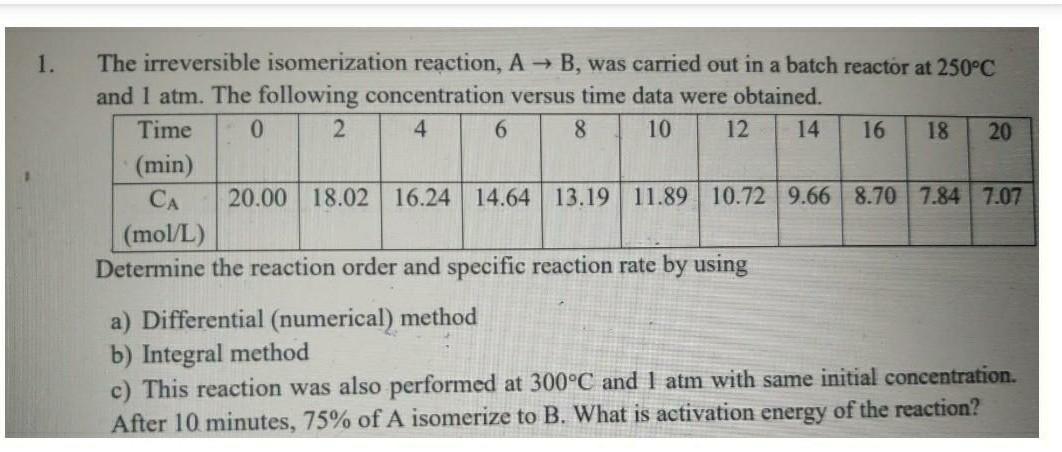
1. The irreversible isomerization reaction, A + B, was carried out in a batch reactor at 250C and 1 atm. The following concentration versus time data were obtained. Time 0 2 4 6 8 10 12 14 16 18 20 (min) CA 20.00 18.02 16.24 14.64 13.19 11.89 10.72 9.66 8.70 7.84 7.07 (mol/L) Determine the reaction order and specific reaction rate by using a) Differential (numerical) method b) Integral method c) This reaction was also performed at 300C and 1 atm with same initial concentration. After 10 minutes, 75% of A isomerize to B. What is activation energy of the reaction
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


