Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please DO QUESTION 2!!!! all parts with FULL STEPS Question 2 The data tabulated below were obtained for the decomposition reaction of a chemical compound
Please DO QUESTION 2!!!!
Question 2 The data tabulated below were obtained for the decomposition reaction of a chemical compound at 50 C. From the amount of product collected, the conversion can be calculated and the data are: 30 24 21 18 15 12 9 6 3 | Time (min) 86.6 79.9 75.5 70.063.355.245.233.1 18.20 Conversion% First order reaction rate formula appeared to describe real reactions rates as: a) If is the conversion fraction and is the time in minutes, what are the unit of the constant ? b) Fit a straight line to the above data to find the regression model constant. c) Use two-point interpolation to find the time of decomposition reaction for 68% conversion. d) Use regression model to find the time of decomposition reaction for 68% conversion. e) Compare and comment on the answers obtained in parts c and d. f) Which method you will use to find the conversion of decomposition reaction after 38 min and why all parts with FULL STEPS 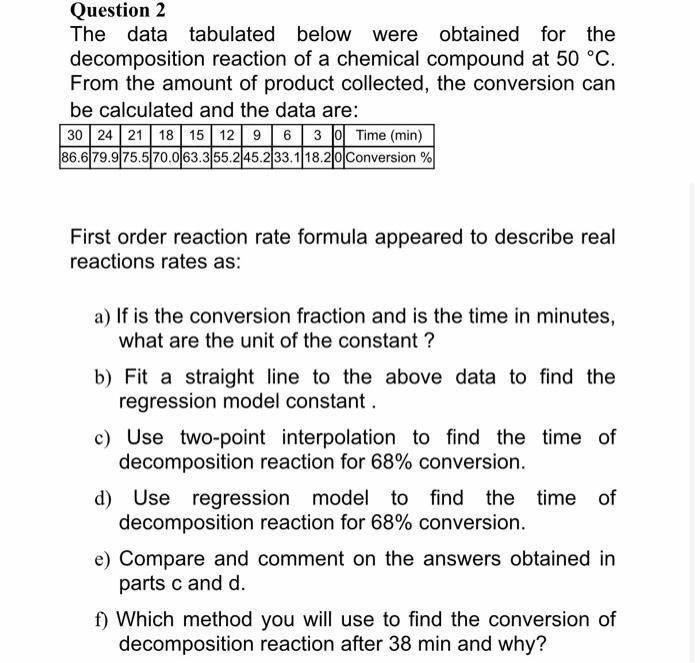

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


