Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please do question 2 mass balances in terms of conversion X. Please show ALL steps and where all information used in calculations is gotten from.
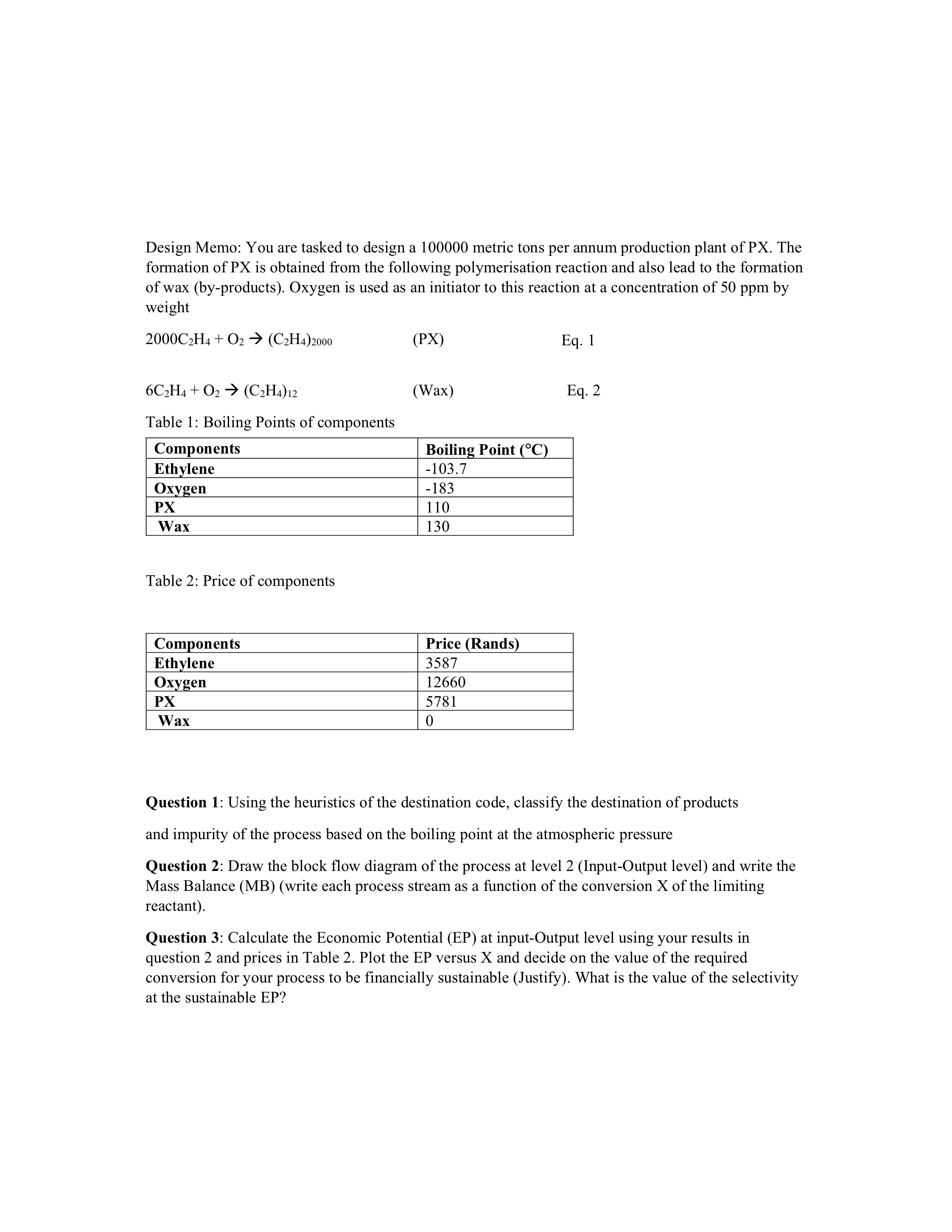

Please do question 2 mass balances in terms of conversion X. Please show ALL steps and where all information used in calculations is gotten from. Prioritise q2/3 if you cant do all the questions as the first questions are needed to do the follow up questions.
Design Memo: You are tasked to design a 100000 metric tons per annum production plant of PX. The formation of PX is obtained from the following polymerisation reaction and also lead to the formation of wax (by-products). Oxygen is used as an initiator to this reaction at a concentration of 50ppm by weight 2000C2H4+O2(C2H4)2000 Eq. 1 6C2H4+O2(C2H4)12 (Wax) Eq. 2 Table 1: Boiling Points of components Table 2: Price of components Question 1: Using the heuristics of the destination code, classify the destination of products and impurity of the process based on the boiling point at the atmospheric pressure Question 2: Draw the block flow diagram of the process at level 2 (Input-Output level) and write the Mass Balance (MB) (write each process stream as a function of the conversion X of the limiting reactant). Question 3: Calculate the Economic Potential (EP) at input-Output level using your results in question 2 and prices in Table 2. Plot the EP versus X and decide on the value of the required conversion for your process to be financially sustainable (Justify). What is the value of the selectivity at the sustainable EP? Question 4: Using the conversion in question 3 finalise the MB at input-output level Question 5: calculate the volume of a PFR reactor (in m3 ) (no pressure drop, Isothermal reaction) to achieve the conversion obtained in Question 3 if the reaction rate for thermal initiation of ethylene is given by: rA=ktCA Eq. 3 Where the rate constant is, kt=8.21010exp[8.314T(84000+47P)] Eq. 4 Where, P=300MPa T=500K (Average temperature where oxygen decomposed) R=8314MPadm3/molK Question 6: By plotting and looking at the Levenspiel Plot, do you think it was justified to use a PFR in question 5 (discuss)? Design Memo: You are tasked to design a 100000 metric tons per annum production plant of PX. The formation of PX is obtained from the following polymerisation reaction and also lead to the formation of wax (by-products). Oxygen is used as an initiator to this reaction at a concentration of 50ppm by weight 2000C2H4+O2(C2H4)2000 Eq. 1 6C2H4+O2(C2H4)12 (Wax) Eq. 2 Table 1: Boiling Points of components Table 2: Price of components Question 1: Using the heuristics of the destination code, classify the destination of products and impurity of the process based on the boiling point at the atmospheric pressure Question 2: Draw the block flow diagram of the process at level 2 (Input-Output level) and write the Mass Balance (MB) (write each process stream as a function of the conversion X of the limiting reactant). Question 3: Calculate the Economic Potential (EP) at input-Output level using your results in question 2 and prices in Table 2. Plot the EP versus X and decide on the value of the required conversion for your process to be financially sustainable (Justify). What is the value of the selectivity at the sustainable EP? Question 4: Using the conversion in question 3 finalise the MB at input-output level Question 5: calculate the volume of a PFR reactor (in m3 ) (no pressure drop, Isothermal reaction) to achieve the conversion obtained in Question 3 if the reaction rate for thermal initiation of ethylene is given by: rA=ktCA Eq. 3 Where the rate constant is, kt=8.21010exp[8.314T(84000+47P)] Eq. 4 Where, P=300MPa T=500K (Average temperature where oxygen decomposed) R=8314MPadm3/molK Question 6: By plotting and looking at the Levenspiel Plot, do you think it was justified to use a PFR in question 5 (discuss)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


