Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please draw structure also! Spectroscopy Unknown. The spectra and data provided were obtained from a pure organic molecule. For 'H NMR Spectra, the integral is
please draw structure also! 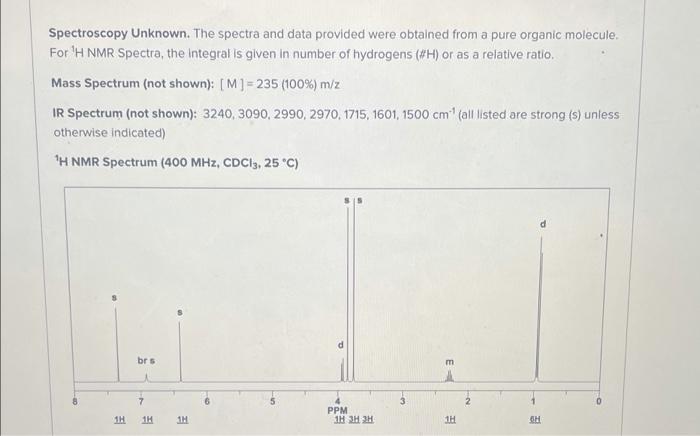
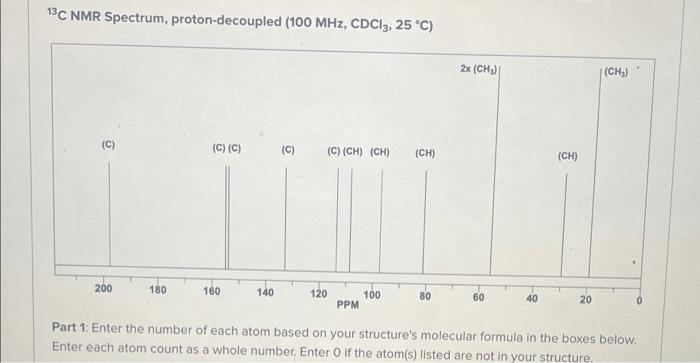
Spectroscopy Unknown. The spectra and data provided were obtained from a pure organic molecule. For 'H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Mass Spectrum (not shown): [M] =235 (100%) m/z IR Spectrum (not shown): 3240, 3090, 2990, 2970, 1715, 1601, 1500 cm (all listed are strong (s) unless otherwise indicated) "H NMR Spectrum (400 MHz, CDC13, 25 C) brs m 6 7 1H 1H 111 PPM 1H 3H2H 1H SH 13C NMR Spectrum, proton-decoupled (100 MHz, CDC13, 25 C) 2x (CH2) (CH) C (C) (C) (C) (C) (C) (CH) (CH) (CH) (CH) 200 180 160 140 120 100 PPM 80 60 40 20 Part 1: Enter the number of each atom based on your structure's molecular formula in the boxes below. Enter each atom count as a whole number. Enter O if the atom(s) listed are not in your structure Spectroscopy Unknown. The spectra and data provided were obtained from a pure organic molecule. For 'H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Mass Spectrum (not shown): [M] =235 (100%) m/z IR Spectrum (not shown): 3240, 3090, 2990, 2970, 1715, 1601, 1500 cm (all listed are strong (s) unless otherwise indicated) "H NMR Spectrum (400 MHz, CDC13, 25 C) brs m 6 7 1H 1H 111 PPM 1H 3H2H 1H SH 13C NMR Spectrum, proton-decoupled (100 MHz, CDC13, 25 C) 2x (CH2) (CH) C (C) (C) (C) (C) (C) (CH) (CH) (CH) (CH) 200 180 160 140 120 100 PPM 80 60 40 20 Part 1: Enter the number of each atom based on your structure's molecular formula in the boxes below. Enter each atom count as a whole number. Enter O if the atom(s) listed are not in your structure 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


