Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please enclose the answers for each question thank you Which of the following statements is false? A. A spontaneous process can occur with a large
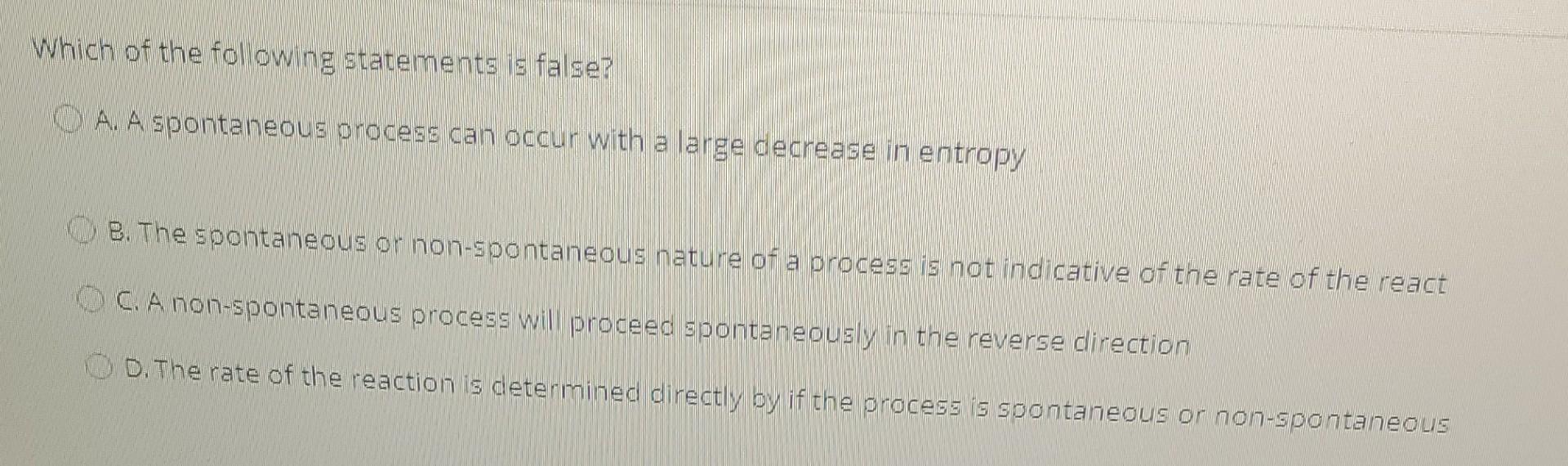


please enclose the answers for each question thank you
Which of the following statements is false? A. A spontaneous process can occur with a large decrease in entropy B. The spontaneous or non-spontaneous nature of a process is not indicative of the rate of the react C. A non-spontaneous process will proceed spontaneously in the reverse direction D. The rate of the reaction is determined directly by if the process is spontaneous or non-spontaneous Calculate how much solid reagent you would need to make 500 mL of a 1M solution of Iris (MW = 121.14 g mol) A. 50.06 3 B. 60.57 8 OC. 80.63 & OD.74.39 8 What is the definition of "functional group? O A. The bonding arrangements within different molecules ** B. A structural unit from which a polymer is built up C. A compound that contains the element carbon D. A portion of a molecule that participates in interactions with other substancesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


