Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please exalain with details, why answer is this?? MISSED THIS? Watch KCV: The integrated Rate Law, IWE: Determining the Concentration of a Reactant If the
Please exalain with details, why answer is this??
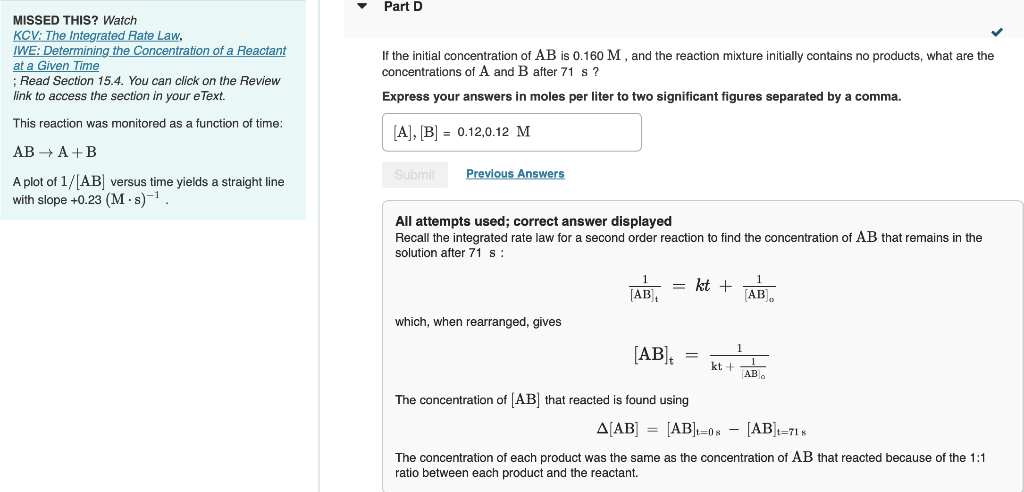
MISSED THIS? Watch KCV: The integrated Rate Law, IWE: Determining the Concentration of a Reactant If the initial concentration of AB is 0.160M, and the reaction mixture initially contains no products, what are the at a Given Time concentrations of A and B after 71s ? ; Read Section 15.4. You can click on the Review Express your answers in moles per liter to two significant figures separated by a comma. link to access the section in your eText. This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope +0.23(Ms)1. All attempts used; correct answer displayed Recall the integrated rate law for a second order reaction to find the concentration of AB that remains in the solution after 71s : [AB]t1=kt+[AB]o1 which, when rearranged, gives [AB]t=kt+[AB]011 The concentration of [AB] that reacted is found using [AB]=[AB]t=0s[AB]t=71s The concentration of each product was the same as the concentration of AB that reacted because of the 1:1 ratio between each product and the reactantStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


